Falmouth Field Trip 2014-
This website reflects the opinions of students and not the views of the University of Southampton or the National Oceanography Centre.
Produced by: Alice Duff, Philippa Fitch, Joanna Gordon, William Harris, Thomas Jefferson,
Eirian Kettle, Jesse Marshall, Dominique Mole, Emma-







Estuary -
Nitrate
Phosphate
Silicon
Dissolved Oxygen
Chlorophyll




Results -
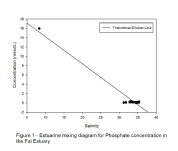
Discussion – Phosphate behaviour is unclear due to the small salinity range at which
the samples were collected. The deviation of the points from the TDL in Figure 1
indicates there may be both addition and removal of phosphate, suggesting behaviour
can be both conservative and non-
Results -
Nitrate profiles (Figure 4) show that nitrate concentrations decreased with depth at all stations except Station 1. Station 7 has the largest range in nitrate concentration (12.7µmol/L).
Discussion – Nitrate behaviour is unclear due to the small salinity range at which
the samples were collected. The deviation of the points from the TDL in Figure 3
indicates there may be both addition and removal of nitrate. Addition of nitrate
has been observed at Station 1. This could be due to river water providing the majority
of biologically important compounds to estuaries (Day et al., 2012). Sewage inputs
are not as important for nitrate compared with phosphate. This is because sewage
is treated for nitrate inputs, but not phosphate, suggesting that nitrate should
behave more conservatively than phophate shown in Figure 3 (Langston et al., 2006).
Stations 2-
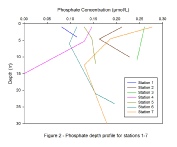
Results – The mixing diagram for silicon (Figure 5) shows no clear behavioural trend in the estuary. Points plot both above and below the TDL with slightly less deviation seen when compared to nitrate and phosphate mixing diagrams. At Stations 3 and 5 silicon profiles are seen to follow the expected trend of decreasing with depth. The remaining stations show an increase in silicon with depth (Figure 6). The largest range in silicon was seen at Station 1 with a change of 1.8µmol/L.
Discussion -
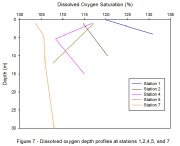
Results –At Stations 1, 2 and 7 dissolved oxygen increases with depth and at Stations 4 and 5 a decrease with depth is seen (Figure 7). The largest range in dissolved oxygen was seen at Station 1 with a change of 11%.
Discussion – Supersaturation is shown at all stations (Figure 7). Stations 4 and
5 are located approximately mid-
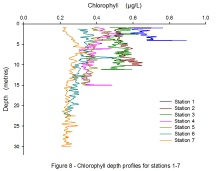
A reducing gradient of chlorophyll can be seen as the sample sites move away from
the riverine end member to the mouth of the estuary, with a mean chlorophyll values
of 0.668v at Station 1 and 0.257v at Station 7. At Stations 1, 3 and 4, peaks in
chlorophyll can be seen between 0-
Station 7, the most saline station sampled, shows the most constant and the lowest chlorophyll values throughout the water column. This is potentially due to the lower turbidity levels at the estuary mouth (4.62 v at Station 7) than at the river source (4.19v) indicating a lower attenuation level and thus a greater likelihood of autotrophs spreading vertically through the water column. The dynamic nature of the riverine end of the estuary is likely causing the greater variation of chlorophyll due to greater temporal and spatial changes of salinity, transmission and nutrient input.
| Geophysics |
| References |
| Introduction |
| Physical |
| Chemical |
| Biological |
| References |
| Introduction |
| Results |
| References |
| Introduction |
| Physical |
| Chemical |
| Biological |
| References |