University of Southampton 2015©


BIOLOGICAL ANALYSIS

GO TO:

SAMPLING AND LABORATORY ANALYSIS:
Water samples were taken through a Niskin bottles using a CTD. The CTD was first
dropped to the near bottom of the sea bed. This gave data on the distribution of
chlorophyll throughout the water column. The sampling depths were then based on the
chlorophyll maximum; samples were taken under the chlorophyll maxima, in the chlorophyll
maxima, and in the water over the chlorophyll maxima. 3 sets of data were collected
at approximately the same longitude and latitude starting at 13:23pm, with ~1 hour
intervals. Samples were then taken to the lab for analysis.

RESULTS:

The CTD data showed the chlorophyll maximum was roughly between 25 and 27 meters. Therefore, it was normal to count a much larger amount of phytoplankton at those depths than above or below. The only depths which seem to be consistent in phytoplankton type are the depth where the chlorophyll maximum was found. After identification through a microscope, it was found that there had been a dinoflagellate bloom of Ceratium fusus; a mixotrophic single celled phytoplankton with sexual and asexual reproduction methods[1]. As can been seen on Figure 1-3, dinoflagellates are the dominant species at the chlorophyll maximum; most - if not all - being Ceratium fusus. Normally, this species of dinoflagellate only blooms in late summer to early autumn[2], but due to previous weather in Falmouth[3], the conditions were optimum for a Ceratium fusus bloom, (as seen in Physical Analysis). Reaching up to 217 cells per ml at station 40, this high cell concentration of Ceratium fusus was observed at the pycnocline, which, offshore, is determined by temperature rather than salinity. Generally, these blooms are initiated after rainfall that causes a drop in surface salinity. This then generates a strong pycnocline closer to the surface which is ideal for these phytoplankton to get their nutrients[4]. Ceratium fusus grow best at water temperatures above 16°c and salinities below 35[4], and can also cause red tides in Eastern Asia regions. However, this species is not known to produce toxins but has been linked to death of invertebrate larvae[5].
As Diatoms are small and fast-reproducing phytoplankton, they were found quite consistently at every depth at each set time interval. Diatoms had the most species richness, especially at the surface with 7 different species per ml at station 41, such as Chaetoceros and 3 different species of Rhizosolenia. Species richness was always highest at the surface, where most light is available (see Irradiance section). Mesodinium rubrum was the only ciliate found randomly at each stations but different depths as seen on Figures 1-3.
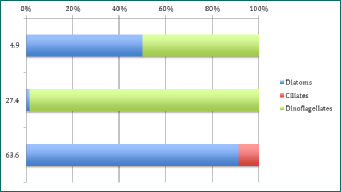

Figure 1 - percentage abundance of phytoplankton types at different depths for Station 40 at 13:23. Y axis is depth in metres.
(Click to expand)
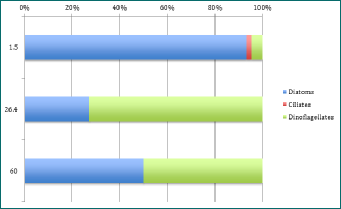

Figure 2 - percentage abundance of phytoplankton types at different depths for Station 41 at 14:41. Y axis is depth in metres.
(Click to expand)
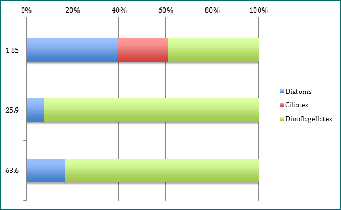

Figure 3 - percentage abundance of phytoplankton types at different depths for Station 43 at 15:23. Y axis is depth in metres.
(Click to expand)

Ceratium fusus
The Ceratium genus is characterized by arms or horns which help them float but also prevent them from moving too quickly. The arms tend to me shorter and thicker in cold salty water, and the opposite in warmer water. C. fusus are 150–230μm in length, 15-30μm in width and are more inclined to grow in warmer waters compared to other Ceratium species. They are fusiform with an apical horn at the end of the epitheca. The hypotheca ends with a fully developed left antapical horn and a reduced right antapical horn, which generally gives a little bend to the organism[5]. It contains numerous yellow-brown chloroplasts but is also mixotrophic[3]. Ceratium fusus is mainly a coastal species but can also be found in some estuaries and further oceanic environments[6].


SAMPLING:
Zooplankton samples were collected at Station B as part of a time series over the period of a day. The first two samples (named Station 40) were collected during a vertical tow of a round closing zooplankton net, with 0.5m diameter and 200µm mesh size. The first sample was collected below the chlorophyll maximum over 13m (at 40m to 27m depth) and the second sample was collected in the chlorophyll maximum, with the tow starting at 30m and ending at 15m depth. The closing mechanism of the net ensured that the samples were not contaminated by organisms from any depths other than those desired. A third, but horizontal, zooplankton sample was collected later in the day (station 44) at the same location, using a bongo tow net. A bongo net consists of two adjacent nets that allow the collection of both, a phytoplankton (mesh size 100µm) and a zooplankton (mesh size 200µm) sample, simultaneously and at the same depth. The duration of the horizontal tow was 10 minutes (311.7m). Again, samples were preserved in formaldehyde and subsequently analysed in the laboratory, where groups/orders were identified and individuals counted. A 10ml sample was analysed for each vertical trawl. However, the horizontal sample was collected within a phytoplankton bloom in the chlorophyll maximum. Consequently, the 2L sample was diluted to a 1:9 sample-water dilute, of which 10ml were analysed. Volume of water sampled and number of individuals per m3 were calculated using the same equations as explained in the Estuary Zooplankton section.
Zooplankton abundance was found to be significantly higher within the chlorophyll maximum (1129.03 number per m3 sampled, Station 40) than below the chlorophyll maximum (254.65 number per m3 sampled), as can be seen in Figure 8. This high abundance of zooplankton coincided with the phytoplankton bloom, and thus chlorophyll maximum, that was detected at around 28m depth, where food availability is high for zooplankton. For more information on the phytoplankton bloom detected, please refer to the Phytoplankton section. Whilst no Copepod Nauplii stages were found below the chlorophyll maximum (see Figure 4), a significant amount was present within the chl maximum zone (see Figure 5). The metabolic advantages of staying within the chlorophyll maximum might support reproduction and survival rate of larval stages, leading to these results[7].
The horizontal tow samples showed similar results of very high zooplankton abundance (8071.90 and 7297.00 number per m3 sampled, for the 100µm and 200µm mesh nets respectively). Furthermore, diversity of zooplankton groups was noticeably higher than in the vertical tow samples (Figures 6-7).

RESULTS:
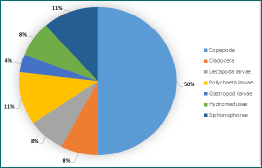

Figure 4 - Zooplankton diversity and abundance at station B at 13:45UTC below the chlorophyll maximum (40-27m). 7 groups of zooplankton were identified in the sample.
(Click to expand)
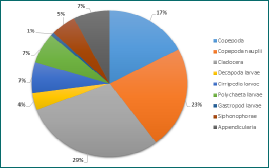

Figure 5 - Zooplankton diversity and richness at Station B at 13:50UTC in the chlorophyll maximum (30-15m). A total of 9 groups were identified.
(Click to expand)
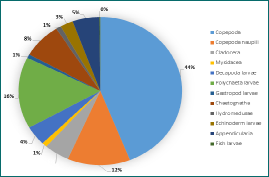

Figure 6 - Zooplankton diversity and abundance at Station B at 16:27UTC in the chlorophyll maximum. A total of 12 groups were found present.
(Click to expand)
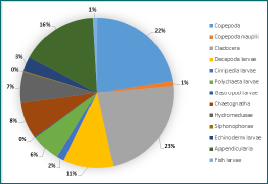

Figure 7 - Zooplankton diversity and abundance at Station B at 16:27UTC in the chlorophyll maximum. 13 groups were found present in the sample.
(Click to expand)
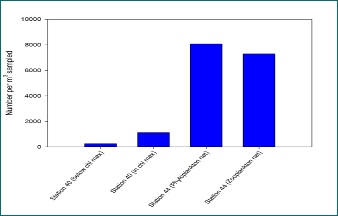

Figure 8 - Comparison of zooplankton abundance in vertical (Station 40) and horizontal (Station 44) tow samples. (Click to expand)

SPECIES IDENTIFIED; MOST ABUNDANT(rollover to expand):

References:
[1] Scott, F. and Brandt, S. Ceratium fusus (Ehrenberg) Dujardin 1841. [Online] Available: http://www.marinespecies.org/aphia.php?p=taxdetails&id=109951. [Accessed: 2015, July 1st] (2011)
[2] Encylopedia of Life (EOL) Ceratium fusus (Ehrenberg) Dujardin 1841. [Online] Available: http://eol.org/pages/899358/overview. [Accessed: 2015, July 1st] (2012)
[3] [Online] Available: http://www.worldweatheronline.com/Falmouth-weather-history/Cornwall/GB.aspx [Accessed: 2015, July 1st]
[4] Baek, S. H., Shimode, S. and Kikuchi, T. Reproductive Ecology of the Dominant Dinoflagellate, Ceratium fusus, in Coastal Area of Sagami Bay, Japan. Journal of Oceanography. 63: 35-45. (2007)
[5] Montagnes, D. Guide to Harmful Phytoplankton. University of Liverpool, UK. [Online] Available: http://www.liv.ac.uk/hab/Data%20sheets/c_fusu.htm. [Accessed: 2015, July 1st] (2006)
[6] Horner, R. A. A Taxonomic Guide To Some Common Phytoplankton. Biopress Limited, Dorset Press, Dorchester, UK. p.200. (2002)
[7] 1. Williamson, C. E. & Sanders R. W. Utilization of subsurface food resources for zooplankton reproduction: Implications for diel vertical migration theory. Limnol. Oceanogr. 41, 224-233 (1996)



These images are not property of group 12.
Copyright belongs to photographers.
This image is not property of group 12. Copyright belongs to photographer.