Offshore: Biological
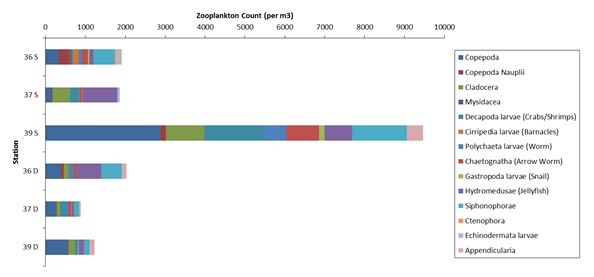
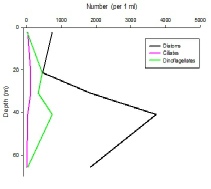
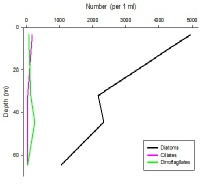
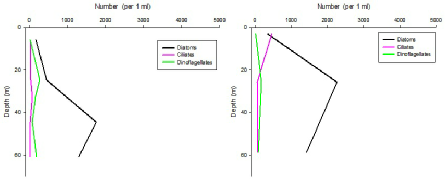

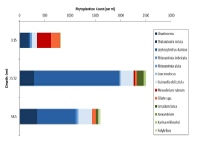
Figure 1. The number of zooplankton individuals in each family present at each station. Here S means surface column from 30 – 0 m, and D means deep column from 55 – 30 m.
The most common zooplankton found were copepods, which were at a more advanced stage than their less abundant juvenile form the copepod nauplii. Although copepods are expected to migrate diurnally and so appear deeper during the day (Daro, 1985), there appeared to be little difference between copepod population size at the two depths. However the nauplii were found mainly in the surface stations, suggesting they had not developed diurnal feeding rhythms (Daro, 1985). No zooplankton family exhibited preference of depth in the water column and no obvious trend in zooplankton numbers occurred across the depths. As zooplankton are motile they can migrate vertically to locate their food and so are not limited to the euphotic zone where phytoplankton are predominantly found. However, this phytoplankton behaviour is not observed in our samples; a chlorophyll maximum was observed below the euphotic zone at ~40 m with a smaller peak at ~20 m at all stations, which does is not mirrored by the zooplankton distribution.
The most prevalent meroplankton recorded were the hydromedusae, whose growth rate is potentially increasing due to the ideal temperatures and large number of copepods present (Matsikis, 1993) and thus creating the bloom of jellyfish observed off Cornwall (BBC, 2014). Other meroplankton, such as polychaete larvae, were high in numbers as the protected SACs (Langston et al., 2003) may provide safe areas for reproduction by benthic adults. The three most uncommon families (Mysidacea, Ctenophora and Cirripeda) were all only found at the surface of Station 36. Mysidacea larvae may be low due to high sensitivity to toxic metals (Cripe, 2009) which are extremely high off the Falmouth shore (Bryan et al., 1987); ctenophores due to lack of they favourite prey, copepod nauplii and ciliates (Stoecker et al., 1987); and cirripedia due to their intertidal and shallow water preference as we sampled 9 miles off shore. There were an unexpectedly high number of zooplankton individuals (~9500) in the surface waters of station 39. This could be anomalous due to individual accuracy of identification; however the proportional abundance of the zooplankton families is similar to the majority of stations.
This web page shows;
Figures 2 -
Discussion
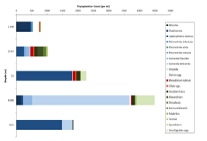
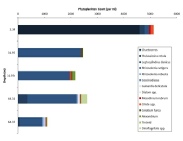
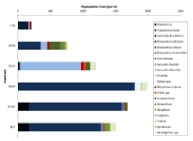
Figures 6 – 9. The number of phytoplankton individuals present in water samples taken from station 36, 37, 39 and 40 respectively. Repeat analysis of a single depth’s sample is indicated by an “r”. Note that blue represents diatoms, red represents ciliates and green represents dinoflagellates. Click on each figure to enlarge.
Across all stations and at nearly all depths diatoms dominate the phytoplankton community.
This is unexpected in the summer stratified waters which usually favour dinoflagellates.
However diatoms tend to dominate in dynamic systems with fresh nutrient inputs, especially
silica which is high in our samples, as it is required to form the external silica
frustule surrounding the cell (Fishwick, 2008). Diatoms decrease from the surface
waters to just below the main thermocline at both stations 36 and 37. Stations 36,
37 and 39 also show a peak at around 40 m as a response to the second smaller thermocline.
Station 40 does not exhibit this second thermocline and hence no diatom peak was
observed. This was confirmed by the lack of backscatter at 40 m recorded by the ADCP
which had been present at the previous stations. There was an unexpected peak of
diatoms in the surface waters at station 37 that was due to an unprecedented amount
of Chaetoceros (~4500 per ml). These were often observed in chains possibly after
a period of exponentially high growth (Ichimi et al., 2012) due to the lowest count
of copepods whose main diet is often Chaetoceros species (Cheng-
The only occasions where this does not occur are the ciliate-
Discussion
References
BBC, 2014. Accessed online at [http://www.bbc.co.uk/news/uk-
Blanco, E . P., Lewis, J., and Aldridge, J., 2009. The germination characteristics
of Alexandrium minutum (Dinophyceae), a toxic dinoflagellate from the Fal estuary
(UK). Harmful Algae 8(3):518-
Bryan, G. W., Gibbs, P. E., Hummerstone, L. G., Burt, G. R., 1987. Copper, Zinc,
and Organotin as Long-
Cheng-
Crawford, D. W., Purdie, D. A., Lockwood, A. P. M., Weissman, P., 1997. Recurrent
Red-
Cripe, G. M., 2009. Comparative acute toxicities of several pesticides and metals
to Mysidopsis bahia and postlarval panaeus duorarum. Environmental Toxicology and
Chemistry 13(11):1867-
Fishwick, J. R., 2008. Biological and photo-
Holligan, P. M., & Harbour, D. S., 1977. The vertical distribution and succession
of phytoplankton in the English Channel in 1975 and 1976. Journal of the Marine Biological
Association of the United Kingdom 57(4):1075-
Ichimi, K., Kawamura, T., Yamamoto, A., Tada, K., & Harrison, P., 2012. Extremely
high growth rate of the small diatom Chaetoceros salsugineum isolated from an estuary
in the Eastern Seto Inland Sea, Japan. Journal of Phycology 48(5):1284-
Jonsson, P., 1989. Vertical distribution of planktonic ciliates – an experimental
analysis of swimming behaviour. Marine Ecology Progress Series 52:39-
Matsakis, S., 1993. Growth of Clytia spp. hydromedusae (Cnidaria, Thecata): effects
of temperature ad food availability. Journal of Experimental Marine Biology and Ecology
171(1):107-
Smyth T. J., Fishwick J. R., Al-
Stoecker, D. K., Verity, P. G., Michaels, A. E., & Davis, L. H., 1987. Feeding by
larval and post-
Widdicombe, C. E., Eloire, D., Harbour, D., Harris, R. P., & Somerfield, P. J., 2009.
Long-
Falmouth, 2014

 Back to top
Back to top
Back to top
Back to top