



Date of data collection: 07/07/17
Location: See map on the left and click buttons below.
Weather Conditions:
Sunny with cloudy intervals,
Maximum temperature:
21°C, average 17°C;
Average Humidity: 82%;
Wind Speed 11km/h
Tide: HW - 10:27 (UTC);
LW – 16:17 (UTC)
Materials and Methods
In order to understand these factors, the data needed to be collected using a variety of equipment which used in successive order for each station. Firstly, a hand-deployed CTD (EXO YSI multi-probe) was used to scope out the water column for the thermocline given by a temperature profile and peaks in turbidity which could suggest a DCM. Turbidity was used as a proxy for chlorophyll concentration after conversion via a quadratic equation based on the correlation between turbidity and chlorophyll.
A Niskin bottle was then deployed to where the DCM appeared to be and the water sample was used to collect chemical data to be analysed in the lab for oxygen saturation, silicate, phosphate, nitrate. Water samples were collected to measure nitrate and phosphate by filtering 100ml of sample from each Niskin bottle sample into a brown glass bottle.
A sample to measure silicate was prepared by filtering 100ml of sample from each Niskin bottle sample into a plastic bottle. A glass bottle was not used as the silicate concentration in the sample could have been altered due to its composition.
To measure oxygen saturation, a glass bottle was filled to the very top with water from the Niskin as any trapped air would affect the oxygen concentration of the sample. Manganese(II) sulfate and alkaline iodide to form a cream precipitate and in which the oxygen was combined. The bottle was closed with glass stopper and then submerged in a bucket of water to totally seal the lid and prevent oxygen escaping. The whole process was carried out very quickly in order to minimise interaction between air and water which would alter the original concentration. A Niskin bottle was also deployed in the surface waters at 2m depth and this water sample was used to prepare the bottles for analysis in the lab in the same way as before.
Biological data about the abundance of phytoplankton was also collected from the Niskin bottle by adding 100ml of the water sample to a glass bottle containing Lugol’s reagent, using a pipette without a filter head. Chlorophyll samples were collected by passing 50ml of a sample through a filterhead from a pipette and then submerging the filter in 6ml of 90% acetone. The samples were frozen for 12 hours before they were analysed in the lab the next day.
The final method of data collection was to measure zooplankton abundance in the area. A net with a 0.5m and 200um mesh size was towed alongside the ship and the sample was preserved with Formalin in a plastic bottle to ensure that no grazing of the organisms occurred before the sample was analysed the next day.
The Terramare was used as the vessel for offshore data collection with the intention of creating a time series data set from a single location through periodic sampling over a period of approximately 6 hours. This would allow an insight to the dynamics of the Deep Chlorophyll Maximum (DCM) due to factors such as irradiance (PAR),and physical properties of the water column such as thermal stratification.
Furthermore, the collection of biological data i.e. phyto- and zooplankton at each station will give an indication of how the variation in physical factors affect their abundance and consequent implications for the wider ecosystem. Due to the present season (summer) it is reasonable to assume that there will be minimal phytoplankton abundance at the surface due to the depletion of nutrients from the spring bloom; therefore, one could expect to see highest abundance at the DCM where nutrients accumulate at the base of the thermocline; forming the nutricline. Irradiance will be lowest here hence the need for specialist phytoplankton to exploit this.
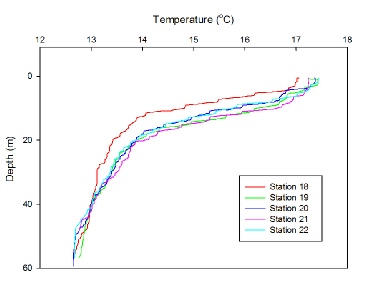
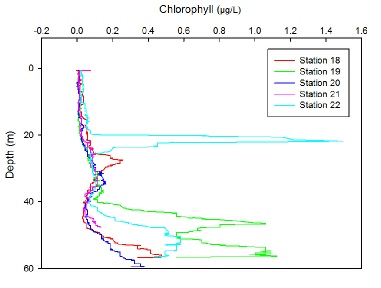
Temperature
Figure 1 displays the decrease in temperature with depth throughout the water column at each time of sampling throughout the day. A clear thermocline is evident at all stations due to the sharp decrease in temperature from ~ 5m to 20m. Temperature at the surface is fairly constant throughout the day (17˚C) showing a slight increase as time increases, due to an increase in solar radiation. A 4˚C temperature change occurs across the thermocline, with all stations showing a homogenous layer of temperature 13 ˚C from 40m and below. At station 18 (09:16 UTC), the thermocline is slightly shallower than all other stations with the base occurring at around 16m. The dynamics of the thermocline are evident from the graph, as the depth and structure of the thermocline fluctuates slightly throughout the day revealing the internal wave structure. All stations apart from 18 experience a further decrease in temperature from the base of the thermocline at 20m until 40m, suggesting a second, weaker thermocline.
Chlorophyll
In shelf seas, when thermal stratification occurs in the summer a deep chlorophyll maximum (DCM) forms (Holligan et al., 1984)- a feature where the surface layer (limited by nutrients but high in irradiance) meets the deeper mixed layer (limited by light but rich in nutrients) forming conditions conducive to phytoplankton growth. The DCM would be expected to occur at the base of the thermocline, roughly 20m for each station, however from figure Y (chlorophyll graph), all stations apart from 22 (14:43 UTC) show a peak in chlorophyll concentration at 25-30m, deeper than expected.
Station 22 shows a prominent increase in chlorophyll from 0.1 to 1.5 mg/L at the base of the thermocline, which extends to 23m indicating the presence DCM, and then decreases again to base level of 0.1mg/L before another peak at 50m.
All stations experience a peak in chlorophyll again at a depth of 55m, particularly noticeable at station 19 (10:48 UTC). These are unlikely to be explained by a DCM as 55m is clearly well below the thermocline when looking again at Figure (temp graph). On the day of sampling, the fluorometer on the CTD was broken hence chlorophyll measurements were calculated using turbidity measurements, as it can be assumed when sampling offshore, most turbidity present in the water column can be explained by phytoplankton and therefore is a proxy for chlorophyll. A polynomial regression was fitted using previous CTD data to turbidity against chlorophyll measurements, which gave an R2 value of 0.94, and so the equation of the line y = 2.1656x2 + 0.3683x was used to convert the turbidity values into chlorophyll (µg/L).
Figure 1: Depth profiles of temperature (˚C) for time series taken at location 50˚06’650’’N, 004˚52’052’’W, Station 18: 09:16 UTC, Station 19: 10:48 UTC, Station 20: 12:20 UTC, Station 21: 13:30 UTC, Station 22: 14:43 UTC.
Figure 2: Depth profiles of chlorophyll (mg/L) for time series taken at location 50˚06’650’’N, 004˚52’052’’W, Station 18: 09:16 UTC, Station 19: 10:48 UTC, Station 20: 12:20 UTC, Station 21: 13:30 UTC, Station 22: 14:43 UTC.
Physical structure & DCM
Chemical Analyses


Figure 3: Contoured time series of phosphate concentration taken at 50˚06’650’’N, 004˚52’052’’W over the course of a 6 hour period on 07/07/17. Samples were taken at Station 18: 09:16 UTC, Station 19: 10:48 UTC, Station 20: 12:20 UTC, Station 21: 13:30 UTC, Station 22: 14:43 UTC. Colours indicate concentration of phosphate in mmol/L.
Figure 6: Contoured time series of % oxygen concentration taken at 50˚06’650’’N, 004˚52’052’’W over the course of a 6 hour period on 07/07/17. Samples were taken at Station 18: 09:16 UTC, Station 19: 10:48 UTC, Station 20: 12:20 UTC, Station 21: 13:30 UTC, Station 22: 14:43 UTC.. Colours indicate % saturation of oxygen.
Figure 4: Contoured time series of silicate concentration taken at 50˚06’650’’N, 004˚52’052’’W over the course of a 6 hour period on 07/07/17. Samples were taken at Station 18: 09:16 UTC, Station 19: 10:48 UTC, Station 20: 12:20 UTC, Station 21: 13:30 UTC, Station 22: 14:43 UTC.. Colours indicate concentration of silicate in mmol/L.
Chlorophyll values were also derived from acetone from niskin bottles at the surface and the predicted depth of the DCM from the turbidity measurements, to see how well the values calculated from turbidity compared to the acetone derived values.
At all stations apart from station 22 (14:43 UTC), the chlorophyll readings from the acetone derived values were at least double those predicted from the turbidity CTD data. At station 20, the chlorophyll measurement derived from acetone at 32m (4.5 µg/L) was drastically different from that predicted from turbidity (0.136 µg/L), indicating that turbidity grossly underestimated the concentrations of chlorophyll at all stations except 22. This could explain why the DCM appeared to be deeper than the thermocline, as in fact the concentration of chlorophyll at the base of the thermocline may have been much higher than indicated from figure (chl graph), and the other stations in fact may have a profile much more like station 22, with the DCM corresponding to the depth of the thermocline.
When interpreting the apparent the spike in chlorophyll at 55m present at all stations, it is also important to factor in the use of turbidity as a proxy, as it is most likely explained not by a DCM as the depth is well below the base of the thermocline where there is enough light for phytoplankton to photosynthesise. It is more likely explained by detritus or marine snow raining from the surface layer, though further investigation is needed to corroborate this.
Nutrients and oxygen profiles
Phosphate (Figure 3) and silicate (Figure 4) both show the same general pattern of low concentrations at the surface which increases with depth.
Phosphate is almost completely depleted in the surface layers at all stations, with concentrations as low as 0.1 mmol/L until a depth of 30m where concentrations begin to increase. A notable increase in concentration to 0.2 mmol/L is evident around 40m suggesting the presence of the nutricline. From 40m, the concentration increases rapidly, with concentrations reaching a maximum at station 22 (14:43 UTC) of 0.4 mmol/L at 45m depth. Lowest concentrations occured at 13:00 UTC of 0.0491 mmol/L. After 13:00 UTC a shallowing of the nutricline is indicated by the shallowing contours to 35m.
Silicate shows a similar pattern, though an increase in surface concentration was observed at station 19 (10:48 UTC) of 0.73 mmol/L which then decreases again by station 20 (12:20 UTC). Surface concentrations are significantly depleted by station 21 (13:30 UTC). Concentration generally increases with depth, with a nutricline evident at 30m at station 18, which then deepens throughout the time series and is no longer evident from station 21 onwards (13:30 UTC). Maximum concentration was observed at 11:00 UTC of 1.2 mmol/L.
Nitrate (Figure 5) follows a different pattern, with high concentrations of 0.8mmol/L at 27m and below at station 18 (09:16 UTC) which then decrease down to 0.4mmol at station 19 (10:38UTC) and then remain low (0.2mmol/L) for the rest of the time series at depth. At station 20 (12:20 UTC) the concentration at the surface has reached its maximum of 0.8mmol/L, decreasing with depth and with time, and by station 21 (13:30 UTC) the surface concentration has fallen again to almost 0 mmol/L.
Percentage oxygen saturation (Figure 6) is generally around 60% for the entire water column, and decreases slowly with depth. An increase in surface oxygen to 80% was observed at station 20 (12:20 UTC), corresponding to an increase in surface nitrate (Figure 5) and following the observed increase in silicate at station 19 (Figure 4), most likely caused by the increase in nutrients stimulating primary production. Oxygen then decreases to 0% at station 21 (13:30 UTC) corresponding with a depletion of surface nutrients (Figure 4 and 5).
The depth profiles of phosphate and silicate can be explained by the stratification of the water column; the surface layer above the thermocline has been depleted of nutrients from the spring phytoplankton bloom, and mixing from below is prevented by the thermal stratification leading to low surface concentrations (Ruardij et. al, 1997). Concentrations are high at depth due to mixing from deep waters and remineralisation. The depth of the nutricline evident from Figure 3 and Figure 4 is deeper than the thermocline, which could be explained by high light penetration leading to production occurring below the mixed layer and so a deeper nutricline in relation to the thermocline (Williams and Follows, 2011). This may also explain the absence of a nitracline (Figure 5) and disappearance of the silicate nutricline from 13:00 UTC onwards, though irradiance data was not obtained on the day of sampling and so this is purely speculation.
Figure 5: Contoured time series of nitrate concentration taken at 50˚06’650’’N, 004˚52’052’’W over the course of a 6 hour period on 07/07/17. Samples were taken at Station 18: 09:16 UTC, Station 19: 10:48 UTC, Station 20: 12:20 UTC, Station 21: 13:30 UTC, Station 22: 14:43 UTC. Colours indicate concentration of nitrate in mmol/L.


Phytoplankton
Figure 7 shows the variation of the five most dominant phytoplankton groups over a period of five hours for the five stations in both surface and deep waters. Deep samples were taken at depths of estimated/assumed Deep Chlorophyll Maximum from the preliminary data given by the CTD profile. The remaining phytoplankton groups that were present in small numbers were grouped into “Other”.
It is clear from the results shown in Figure 7 that Pseudo-nitzchia is the most dominant phytoplankton at all sites, with notable increases at depths 27m at Station 18 and 32m at Station 20, along with an overall increase of all phytoplankton at these stations. Interestingly, the most abundant phytoplankton groups are those that have potential to cause harmful algal blooms (HABs) i.e. Pseudo-nitzchia, Rhizoscleniaceae. Phytoplankton that were grouped as “Other” included notable species such as Mesodinium rubrum which is a ciliate that also caused red tide HABs (Gustafson, 2000).
The increases in phytoplankton correlate with the nutrient concentrations (see chemistry section above). For example at station 18, a nutricline is present at 30m which corresponds to the high phytoplankton abundance at 27m.
Parsons & Dortch (2002) found that Pseudo-nitzchia growth is disproportionally greater than other diatoms – explaining why it is found in such high abundance compared to similar diatoms found at the same site.
The dominance of these pennate and chain forming diatoms (Pseudo-nitzchia, Rhizoscleniaceae) could explain the accumulation of silicate at depth; from the remineralisation of dead individuals that have sunk down.
Zooplankton
Figure 8 exemplifies the increase in phytoplankton through the subsequent increase in zooplankton. In Figure 7, a significant spike of phytoplankton at Station 18, depth 27m has a mirroring zooplankton spike, and further at midday during peak PAR there is the above-mentioned increase at Station 20, depth 32m which can be seen to reverberate through the community with significant zooplankton increases afterwards in the deep water of Station 21 and 22. This gives a good indication that the Deep Chlorophyll Maximum is occurring at these depths (20-30m) as shown in Figure 7 due to the high abundance of phyto- and zooplankton.
Additionally, the majority of all stations were dominated by Copepoda and Copepoda nauplii, which gives stronger evidence for the dominance in the bloom structure as there have previously been links between species such as Pseudo-nitzschia and Copepoda; to the extent that Tammilehto (2015) suggests a positive correlation between Copepod presence and enhanced toxin production from Pseudo-nitzfschia.
Biological Analyses
Figure 7: Number of phytoplankton cellsL-1 sampled at location 50˚06’650’’N, 004˚52’052’’W over the course of a 6 hour period on 07/07/17. The five most dominant groups are displayed. Times are displayed atop the bars and depth of sample along the x axis. 5 different stations were sampled at different times throughout the day- Station 18: 09:16 UTC, Station 19: 10:48 UTC, Station 20: 12:20 UTC, Station 21: 13:30 UTC, Station 22: 14:43 UTC.
Figure 8: Total number of zooplankton cellsm-3 sampled at location 50˚06’650’’N, 004˚52’052’’W over the course of a 6 hour period on 07/07/17. Times are displayed atop the bars and depth of sample along the x axis. 5 different stations were sampled at different times throughout the day- Station 18: 09:16 UTC, Station 19: 10:48 UTC, Station 20: 12:20 UTC, Station 21: 13:30 UTC, Station 22: 14:43 UTC.
References
Gustafson, D. Stoecker, D. Johnson, M. Van Heukelem, W. and Sneider, K., 2017, Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum., Nature, 405, 1049 – 1052.
Hilton, J. and Phillips, G., 1982, The Effect of Boat Activity on Turbidity in a Shallow Broadland River., The Journal of Applied Ecology, 19, 143.
Parsons, M. and Dortch, Q., 2002, Sedimentological evidence of an increase in Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal eutrophication., Limnology and Oceanography, 47, 551 – 558.
Ruardij, P., Van Haren, H. and Ridderinkhof, H., 1997, The impact of thermal stratification on phytoplankton and nutrient dynamics in shelf seas: a model study. Journal of Sea Research, 38, 311 – 331.
Tammilehto, A., Nielsen, T., Krock, B., Møller, E. and Lundholm, N., 2015, Induction of domoic acid production in the toxic diatom Pseudo-nitzschia seriata by calanoid copepods., Aquatic Toxicology, 159, 52 – 61.
Williams, R. and Follows, M., 2011, Ocean dynamics and the carbon cycle., Cambridge: Cambridge University Press, 268.
DISCLAIMER: The findings and views expressed on this website are not those of the University of Southampton or the National Oceanography Centre