
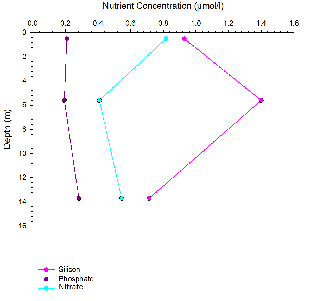
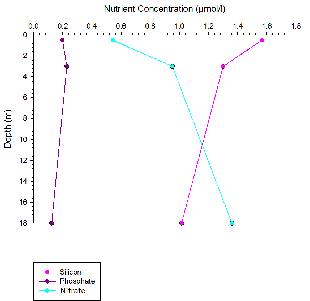
Chemistry analysis
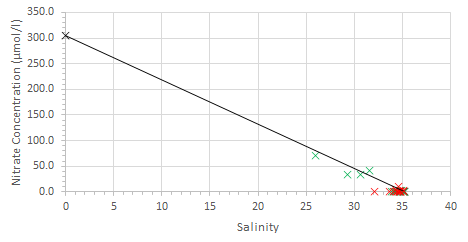
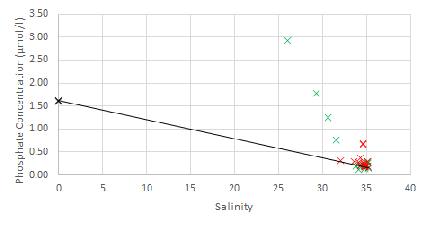
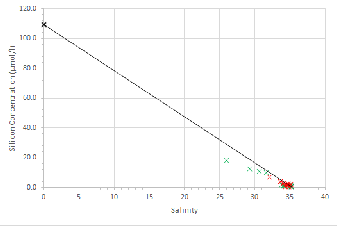
Estuarine mixing diagrams are primarily used to indicate the extent of physical mixing of fresh and saline waters of different compositions along salinity gradients. If there are no biogeochemical processes occurring within the estuary then mixing results in a linear relationship between the two endmembers, this is known as a theoretical dilution line (TDL) (Chester, 2000).
Mixing diagrams assume that the estuary is in a steady state, there is only one freshwater and one saline endmember and that the concentration of the endmembers is constant on a timescale greater than the residence time of the water in the estuary. They also assume that there are no additional inputs. The River Allen was used as the freshwater endmember, whilst the Fal estuary has six main tributaries and 28 minor creeks it can be assumed that these have a negligible influence on the nutrient concentrations and mixing of fresh and saline water within the estuary (Langston et al., 2003).
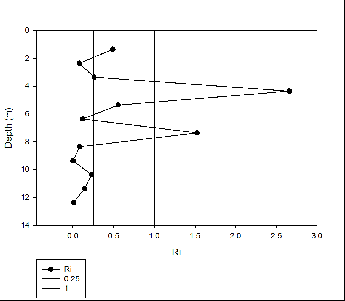
Figures 8 -
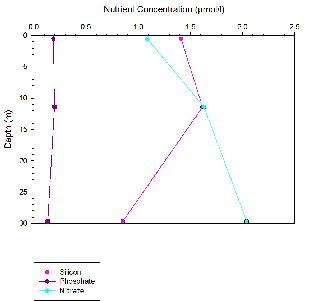
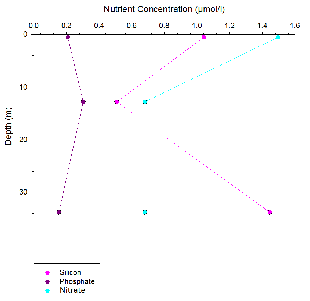
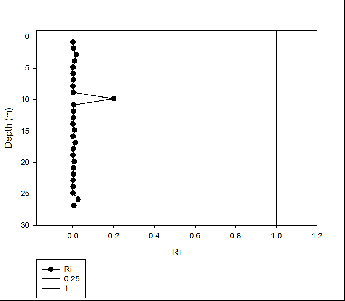
Silicon appears to behave non-
For phosphate non-
Nitrate behaved conservatively this is due to non-
CTD Profile Analysis
CTD profiles were taken at four stations in the lower estuary (Stations 18-
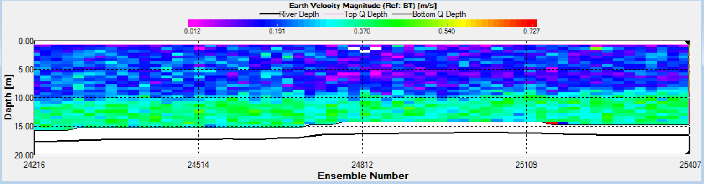
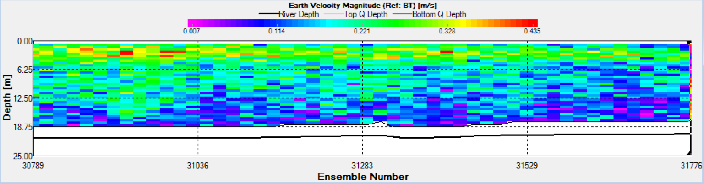
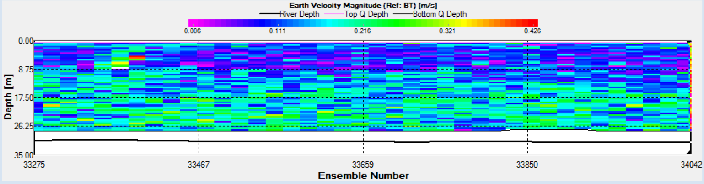
On the turn of the tide three ADCP transects were taken to form a triangle to observe
the water movement. The triangle shows that the tide was still flooding in the centre
of the channel however it had begun to ebb along the banks of the estuary. This can
be seen by the East-
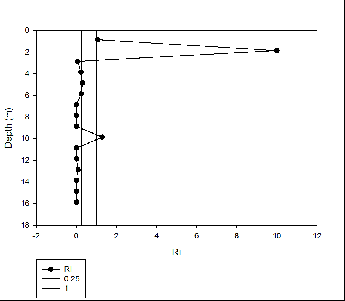
At all four stations the phosphate concentration was significantly lower than the
silicon and nitrate concentrations. The fine structure of the nutrient profiles with
depth is lost as sampling was only taken at the surface and bottom of the water column
as well as at a mid-
Station 18: 50 12.176 N, 005 002.411 W. 12:36 UTC
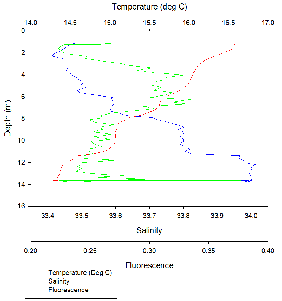
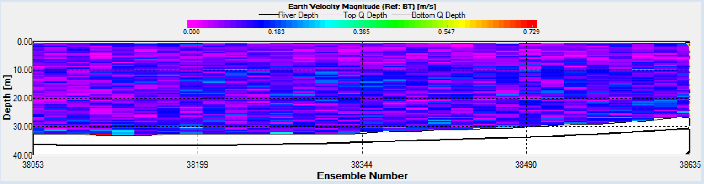
Station 18 was situated at 50 12.176 N, 005 002.411W as seen in Figure 2. ADCP measurements show that the fastest flow occurred at depth near the seabed. (Figure 14).
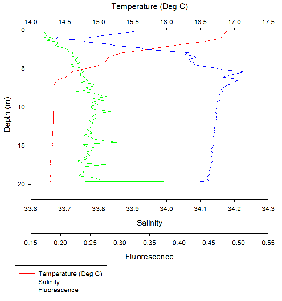
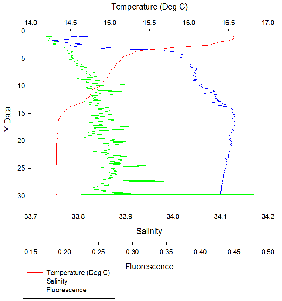
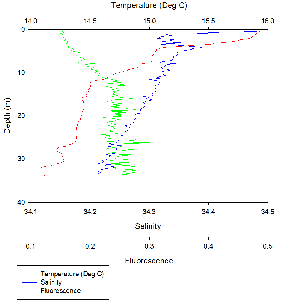
The water column is stratified with warm, less dense freshwater overlying denser,
cooler saline water. This is typical for an estuarine environment. There are two
peaks in fluorescence, one between 4-
Fluorescence peaked at 4-
Station 19: 50 11.452N, 005 002.755 W. 13:42 UTC
Station 19 was situated at 50 11.452 N, 005 002.755 W as seen in Figure 2. ADCP measurements
combined with the CTD profile shows that there was a layer of fast flowing warm less
saline water between 1-
There was a distinct thermocline and halocline at around 5 m, resulting in a highly
stratified surface layer and a well-
Station 20: 50 10.741 N, 005 001.603 W. 14:03 UTC
Station 20 was situated at 50 10.741 N, 005 001.603 W as seen in Figure 2. ADCP measurements show that the fastest flow occurred at depth near the seabed. (Figure 21).
Like station 19 there was a distinct thermocline and halocline at around 5 m, resulting
in a highly stratified surface layer and a well-
Station 21: 50 08.666N, 005 01.435W. 14:52 UTC
Station 21 was situated at 50 08.666 N, 005 01.435 W as seen in Figure 2. ADCP measurements show that flow remained fairly constant with depth. ADCP transect 4 shows that the tide was beginning to turn as it was still flooding in the centre of the channel where the station was situated but had started to ebb along the banks of the estuary (Figure 25).
Like stations 19 and 20 there was a distinct thermocline and halocline at around
5 m, with phosphate concentration remaining fairly constant with depth. Fluorescence
increased with depth until around 12 m and then remained fairly constant, this coincided
with an increase in chlorophyll concentration from 1.0-
At the surface there was a layer of warm saline water overlying cold fresh water;
since this is still an estuarine environment, it is expected that cool fresh water
would be at the surface. As the station was situated in the mouth of the estuary
near Black Rock, this feature could be due to the incoming flood tide trapping fresh
water into surrounding bays at the mouth of the estuary. The CTD was taken on the
turn of the tide as observed in Figures 2d -
|
Date |
06/07/2017 |
|
Time on Station (UTC) |
07:50 - |
|
Location |
50°14.52N, 5°02.85W to 50°14.96N, 5°02.20W |
|
Weather |
Sunny (0/8) Sea state: 1 Temperature: 17 - |
|
Wind (mph) |
14 (North) |
|
Water Depth (m) |
0.5 - |
|
Pasty |
Pasty Co. (1/5): Recommended: Don’t Go! (Too Salty) |
Introduction
The aim of the estuary practical was to look at the variation of nutrients with varying
salinities along the whole estuarine system in the River Fal.
The experimentation on the river was split between two boats. The Winnie the Pooh collected the shallow estuary data from The King Harry Ferry (50°21.63N, 5°02.66W), up past Malpas towards Truro (50°14.96N, 5°02.20W). RV Bill Conway was used to survey the deeper part of the channel from The King Harry Ferry up to the mouth of the Estuary at Black Rock (50°14.52N, 5°02.85W). ADCP transects were taken across the estuary at points where other riverine inputs were flowing into the main Estuarine Channel. (See Figure 2).

Methods
Niskin Bottle Deployment
A horizontal niskin bottle was deployed off the side of the boat and a sample was taken approximately 30cm beneath the water’s surface by dropping the weight on the closing mechanism. The sample was brought to the surface, then the water used to rinse the probe and two sample containers. The containers were then used to collect two samples, one for chemistry preparation and the other was used with the probe to record pH, temperature and salinity at each location, before being filtered for chlorophyll preparation.
Chlorophyll Preparation
The sample was filtered through a glass fibre filter for each station, and the filter was then added to 6ml of 90% acetone. This was repeated 3 times for each station and the tubes were inverted ready to chlorophyll analysis back in the lab.
The vile numbers and corresponding locations were recorded.
Chemistry lab methods
C hlorophyll a sample processing
hlorophyll a sample processing
The chlorophyll a samples collected off the Pontoon, Winnie the Pooh & Bill Conway on 06/07/2017 and were processed on 07/07/2017 using a fluorometer. The fluorometer was used to calculate the chlorophyll a concentration in the water samples by generating a reading based on the fluorescence of the sample. This reading (µg/L) was recorded and inputted into an excel spreadsheet that includes an equation multiplying the water sample fluorescence readings by the volume of acetone used (6 ml) over the volume of sea water (50 ml). The end result of this equation gave the chlorophyll a concentration in the water samples (µg/L).
Nutrient & oxygen sample processing
The concentrations of nutrients & oxygen were
calculated using standard methodology:
Phosphate and Silicate: Parsons et al.,1984.
Oxygen: Grasshof et al., 1999.
Nitrate (by flow injection analysis): Johnson et al.,1984.
Zooplankton
Zooplankton composition is heavily copepod dominated in the upper, less saline, parts of the estuary. In the mouth of the estuary by Black Rock the zooplankton has a mixed population ofCladocera, Hydromedusae and Copepoda. A variety of other species were also found in low abundance (see Pie Charts). It is to be expected with the increased nutrients from riverine input. The lack of fish larvae indicated that the nutrient rich estuary is not being used as a nursery ground for the early life stages of fish. This could be due to poor water quality which is low in nitrate and phosphate along the whole estuary.
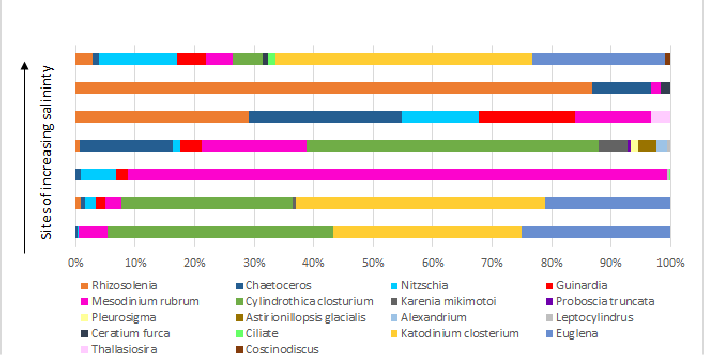
Phytoplankton
There is a greater abundance of phytoplankton in the fresher water located further
up the estuary. This is likely due to the increased input of nutrients at this location,
in conjunction with there being greater light availability here, as these favourable
conditions are able to support heightened planktonic growth. Mesodinium rubrum is
a species that was continually present along the range of salinities, this is concurrent
with the knowledge of this species as it is a euryhaline. The greatest population
of rubrum was found in the mid salinities. Katodinium closterium was only found in
high salinity water, with large abundance. The indication is that closterium is also
a euryhaline but is outcompeted in the mid-
Disclaimer: All the opinions expressed in this site are that of Group 2 and not necessarily the University of Southampton or the National Oceanography Centre.
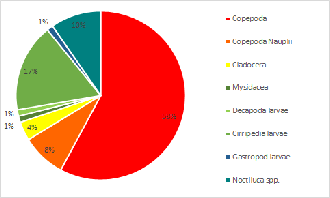
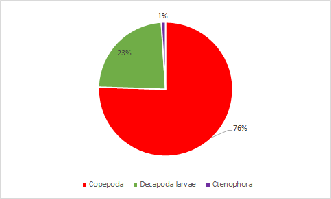
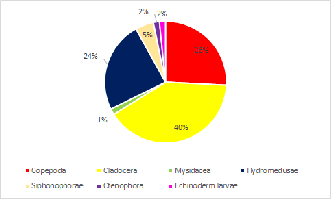
Click charts to enlarge
Estuary
Conway

Winnie the Pooh

Click charts to enlarge
Figure 1 a): Stars at which points a CTD and Niskin Bottle Rosette were deployed in the Fal Estuary on 06/07/17 off the back of R.V. Bill Conway.
Figure 1b): Points indicting where water samples were taken in upper Fal Estuary and Truro River on vessel Winnie the Pooh.
Figure 2: Green Pins represent the start of an ADCP Transect across the estuary. Red indicates the end point. The Point Markers are the track of zooplankton net trawls in the upper estuary and across the mouth of the estuary. The pink triangle shows the track of the ADCP around the mouth of the Percuil River to the East, the Carrick Roads to the South and the remaining rivers to the North West.
Click on transect lines to view ADCP ship tracks & contour plots.
Chemistry Preparation
Lugol’s bottles
100ml of the sample was measured out using a syringe and measuring cylinder, previously rinsed out using the sample water. The 100ml is then added to a lugol’s bottle containing iodine, which stains the phytoplankton within the bottle.
Silicon bottles
100ml of the sample is measured out using a syringe and measuring cylinder and filtered. The bottle is then rinsed in the filtered sample, and 100ml is added to the plastic bottle.
Phosphorous/Nitrate bottles
100ml of the sample is measured out using a syringe and measuring cylinder and filtered. The bottle is then rinsed in the filtered sample, and 100ml is added to the plastic bottle.
The numbers of all the bottles and the stations they correspond to were recorded.
How we chose our Winnie the Pooh stations
The tides were important when considering our locations. Since we experienced low tide when on the boat (at 9:40 UTC), it was not possible to go further than Malpas until later in the day, therefore, we chose to take the first sample at Malpas, where the salinity was as low as we could currently reach, then heading back downstream, two samples were taken equidistant apart, being careful not to sample too close to tributaries entering the river system which would distort the data. At approximately 11:00 UTC, we headed as far upstream as possible and sampled there, finding a salinity of 25.95. Here, however, there were other inputs which needed to be considered during analysis, such as the sewage treatment input.
Click charts to enlarge


Click charts to enlarge
Click charts to enlarge
Click charts to enlarge
Click charts to enlarge
| About Us |
| Overview of Falmouth |
| Biology |
| Physics |
| Chemistry |
| Physics & Chemistry |
| Biology |
| Physics & Chemistry |
| Equipment |
| Vessels |
| References |
