Group 2 Plymouth Field Course












BIOLOGY
A plankton net was lowered over the side of the boat with a collection bottle attached to the end. The net was raised through a chosen depth and the sample was collected. 1 litre of this seawater was set in formalin. 10 ml was measured out for each repeat. 5 ml at a time were pipetted into a Bogorov chamber and the amount/species of zooplankton were counted using a microscope. This process was repeated for all the samples. Then the number of zooplankton per m³ was calculated using the number of zooplankton in a 10ml sample and the volume of sea water sampled. The volume of seawater sampled can be calculated by the equation: ,where R is the radius of the plankton net opening, L is the distance the net was towed and V is the volume.
The depths sampled on the Callista were 0-






Seawater is collected at chosen depths in Niskin bottles on a CTD rosette. 100 ml of each sample was collected and 1 ml of iodine was added to kill the phytoplankton cells. The sample was left overnight so the phytoplankton could bind to the reagent and settle to the bottom and it was then stained with Lugol's iodine. The top 90 ml of seawater was removed using a vacuum pump to leave a concentrated 10ml sample. 1 ml of this solution was then pipetted onto a slide and placed under a microscope. The amount and type of species was recorded for 100 squares. This number is then multiplied by 10 to give the phytoplankton count per ml
The zooplankton count for C5 0-
Between 20-
Fig 2 A -
Fig 2 C -
Fig 2 B -
Fig 1 A -
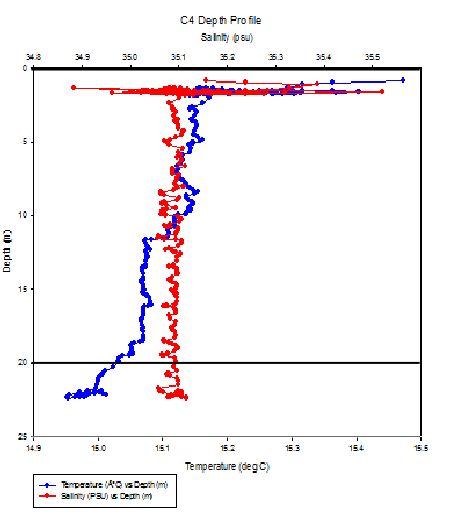
Fig 1 B -
|
Station |
Depth (m) |
Average Number of Zooplankton per m³ |
|
C4 |
0- |
262 |
|
C5 B |
0- |
140 |
|
C5 A |
20- |
168 |
Table 1 -
The zooplankton count for C4 demonstrates that Cirripeda and Copepoda larvae dominate the zooplankton community at this site (figure 1A) with 31 and 10 individuals per 10ml of seawater sample retrospectively. The temperature and salinity profile shows that the water was fairly homogenous above 20m where our sample was taken (figure 1B). C4 had a higher number of individuals per 10ml of sample compared to C5 samples, with 72 individuals compared to 66 and 11 per 10 ml. This difference could potentially be due to location, there may be increased nutrient inputs fuelling increased phytoplankton growth which can support a larger zooplankton community.
a
b
c
d
e

The of species diversity between stations but all were dominated by diatom species.
C1 was dominated by Chaetoceros diatoms (Fig 4A). C3 had a greater variety of species
but once again was dominated by diatoms, Chaetoceros chained diatoms in particular
(fig 4b). The C4 sample only comprised of diatoms and dinoflagellates (fig 4c). C5
was dominated by Chaetoceros Psuedo-
The distribution of phytoplankton density suggests that phytoplankton abundance peaks at 20m (fig 3) for most stations. For example, C5 density peaks at 20m with 2510 cells per ml of sample compared to 410 cells per ml at 5m depth. C3 and C7 also show an increased density at 20m with cell counts reaching 1630 and 230 cells per ml retrospectively. C6 shows a decrease in density below 20m, from 1180 to 40 cells per ml, which may be due to insufficient irradiance to photosythesise and grow. The temperature and salinity profile showed 20m was where the thermocline is (see offshore physics page). This peak of abundance at the thermocline suggest this is a Deep Chlorophyll Maximum Bloom. These are found globally in our oceans and are where the phytoplankton are able to access the nutrient rich waters below the thermocline but still have sufficient sunlight to photosynthesise (Huisman et al., 2006). The lack of sampling throughout the water column, particularly at stations C1 and C4 means that this theory cannot be confirmed at all the stations.
Phytoplankton density varied between stations (fig 5). C5 had the highest abundance
with a total average cell count through the water column of 1540 cells per ml of
sample. C4 had the lowest total average cell count, with only 50 cells per ml of
sample. The variation in phytoplankton cell density could be caused by variation
in terrestrial and riverine inputs, essential sources for nutrients needed for phytoplankton
growth (Tyrrell, 1999) and so can support a larger community. Stations 1-
Fig 3 -
Fig 4 -
f
Fig 5 -
References
Cantin, A., Beisner, B., Gunn, J., Prairie, Y. and Winter, J. (2011). Effects of
thermocline deepening on lake plankton communities. Canadian Journal of Fisheries
and Aquatic Sciences, 68(2), pp.260-
Highfield, J., Eloire, D., Conway, D., Lindeque,
P., Attrill, M. and Somerfield, P. (2010). Seasonal dynamics of meroplankton assemblages
at station L4. Journal of Plankton Research, 32(5), pp.681-
Huisman, J., Pham Thi, N., Karl, D. and Sommeijer, B. (2006). Reduced mixing generates
oscillations and chaos in the oceanic deep chlorophyll maximum. Nature, 439(7074),
pp.322-
Nielsen, T. and Richardson, K. (1989). “Food chain structure of the North Sea plankton
communities: seasonal variations of the role of the microbial loop”. Marine Ecology
Progress Series, 56, pp.75-
Tyrrell, T. (1999). The relative influences of nitrogen and phosphorus on oceanic
primary production. Nature, 400(6744), pp.525-
Raven, J. A. (1983). “The Transport and Function of Silicon in Plants”. Biol. Rev.,
58. pp179-
Yool, A. & Tyrell, T. (2003). Role of Diatoms in Regulating the Ocean’s Silicon Cycle. Global Biogeochemical Cycles, 17(4)
The views and opinions expressed are those of the individual and not representative of the University of Southampton or the National Oceanography Centre.