|
INTRODUCTION
Welcome to the website of Group 10 for the Falmouth
Field-course. The results of our research into the
marine and estuarine environment around Falmouth are
presented on this page. The field-course took place from
26th June-7th July 2012. (All times in UTC)
COURSE AIMS
The aim is to investigate the unique physical,
biological and chemical properties of the Fal estuary
through a variety of techniques. The properties of the
water column both within the estuary and offshore will
be examined along with, how the Fal estuary acts as a
transition zone between freshwater inputs and salt water
and also, how vertical mixing processes offshore
influence the distribution of plankton communities.
Furthermore, a geophysical survey of Carrick Roads will
be conducted to gain an idea of its benthic communities.
ABSTRACTS
OFFSHORE
BLACK ROCK
At station 1 at Black Rock it was found that the Oxygen
saturation profiles decreased from 290% saturated at 2m
to 270% saturated at a depth of 10m. Silicon levels also
decreased from a concentration of 1.02µmol/l at 2m depth
to 0.79µmol/l at 10m Phosphate levels showed a relative
increase from 0.0370µmol/l at 2m depth to 0.067µmol/l at
10m. The salinity did not vary greatly with depth
however the surface temperature rapidly decreased from
13.40⁰C to 12.93⁰C between 2.5m and 3.80m then slowly
decreased to 12.75⁰C at 23.59m. This weak seasonal
thermocline may have occurred due to deep waters and
relatively low tidal mixing (Mathews, 1911). The
recorded fluorescence fluctuated greatly with depth but
generally remained within the range of 0.11 and 0.15mg/m3,,
which can be used as an indicator of phytoplankton
growth supported by the chlorophyll and nutrient
profiles (Hooligan & Harbour, 1977). The turbidity in
the water column increased rapidly within the first
0.52m depth and then remained at a constant value around
3.89% transmission until 27m.
STATION 2
The breakdown of stratification over the course of the
sampling period is further supported by the general
increase in Ri number, which indicated that the water
column is unstable and encourages mixing of previously
stratified layers. Transmission increased rapidly in the
top ~0.5m up to approximately 3.5% for all times at
Station 2 and then remained steady with depth whilst
fluorescence remained between 0.08 V and 0.130 mg/m3.
This variation may have been due to small scale temporal
and spatial changes in currents and nutrient
availability. The phytoplankton were found to be not
confined to the thermocline due to light levels in the
shallow water therefore it was favourable for the
plankton to exist in the more nutrient rich water of the
denser, saline water. The tide was in flood during
sampling which was reflected in the stratified system.
The salinity gradient was layer of fresh water overlying
more dense saline water and the temperature gradient
exhibited a sharp drop in temperature in the same
location as the halocline. As water entered the harbour,
the stratification broke down, seen from 0945 until 1145
when temperature decreased steadily. Throughout the time
series there was increased salinity and decreased
temperature with depth, due to cold, saline, and denser
water (than freshwater) flowing in from offshore.
ESTUARY
The estuary acts as a transition zone to the ocean with
observable gradients for a variety of variables.
Generally salinity increased from station2 to station 7,
whereas temperature decreased.
Throughout the transect there were clear changes in the
chemical, biological and physical components of the
estuary. It was found at station 1 there was a much
greater turbidity (with an Attenuation coefficient of
2.88m-2) than at station 7 (k=0.50m-2).
The suspended material was most likely removed by the
biological activity of zooplankton, whose abundance
increased significantly towards the saline end of the
estuary as well as chemical processes in the water
column. Throughout the transect oxygen saturation was
typically greater than 90%, and an oversaturation
occurred at station 5 as values exceeded 100%, and were
even as high as 104%. Here there is a peak in
chlorophyll concentration of 6.08µg/l therefore
indicating a bloom of phytoplankton. The chlorophyll
concentration was highest at station 7, however there
appeared to be no clear trend between chlorophyll
concentration and the position along the estuary.
GEOPHYSICS
Generally it was observed, by using a drop camera, that
on the east side of the Carrick Roads section of the Fal
estuary there was a high abundance of maerl, with very
little exposed sediment. In contrast, the west side had
no maerl but a higher species diversity, including large
amounts of seagrass as well as larger regions of exposed
sediment.
THE LOCATION
PHYSICAL PROPERTIES AND FORMATION OF THE ESTUARY
The Fal estuary is located in the south west of England
in Cornwall. It is England’s deepest harbour and is the
third largest natural harbour in the world with depths
of up to 34m (Cycleau.com, 2004). At the entrance to the
estuary spring tides have a macrotidal range of up to
5.3m. However, moving closer to the riverine end of the
estuary, a mesotidal range of 3.5m emerges (Pirrie et
al.). This change in tidal range within the estuary
along with tidal currents of up to 2knots results in a
well-mixed estuary.
Fal estuary is a drowned river valley or ria. It was
formed through the combination of eustatic sea level
rise and isostatic recovery of tectonic plates. The
melting of glaciers covering Scotland caused tectonic
uplift and led to a sink in land towards the south of
England, sea levels have also risen around 125m in the
last 180,000 years. The catchment area for the estuary
covers 346km2 (Cycleau, 2004) and multiple rivers.
Many important habitats exist throughout the estuary,
for example, muddy, sandy and rocky sublittoral
(1736ha), intertidal mudflats (653ha) and salt marshes
(93ha). It is also home to many species of scientific
interest, for example the calcareous algae,
Maerl.
For these reasons, along with the high levels of
pollutants, the European Environment Council has
designated Fal estuary as a ‘Special Area of
Conservation’ and a ‘Site of Special Scientific
Interest’.
ANTHROPOGENIC FACTORS
The Fal estuary has become one of the most polluted in
England. Tourism, watersports and boat traffic all
influence this. However, the estuary’s mining history
far exceeds all other impacts on the concentration of
pollutants. The surrounding land is rich in ores,
particularly those of tin and copper which have been
mined through streaming since the Bronze age and in deep
mines in the area since as early as the 18th Century
(Bryan, G.W. and Gibbs, P.E, 1983). Due to the
semi-enclosed nature of the estuary, pollutants become
trapped and concentrated to extents that are seen in
open water. These heavily influence the biology and
chemistry of the water, for example the Wheal Jane
incident of 1992 created toxic copper levels in
Restronguet creek and led to a more resistant species of
N. diversicolor compared to elsewhere in the
estuary. Furthermore, high concentrations of TBT (tributyltin)
in Carrick roads have been shown to cause imposex in Dog
whelks (Nucella lapillus).
GENERAL METHODS
FIELD METHODS
Using an ADCP, information on the velocities,
backscatter and depth of the water column was obtained.
The ADCP ran continuously throughout each sampling
period.
Physical parameters of the entire water column were
obtained using a CTD and fluorometer mounted in a
rosette sampler. Data for the following physical
parameters was collected continuously with depth;
temperature, salinity, fluorescence, light transmission,
irradiance.
Water samples were taken using Niskin bottles set in the
rosette sampler. The depths at which Niskin bottles were
fired were determined by looking at the downwards CTD
profile and selecting any points of interest based on
the physical structure of the water column. This was
often at depths of fluorescence peaks, within layers of
fresher surface water, or above, below and at the
thermocline. These water samples were obtained in order
to quantify (following on-shore lab analysis); dissolved
silicon, dissolved oxygen, chlorophyll, nitrate and
phosphate, phytoplankton abundance and species
distribution.
A zooplankton trawl (with 48.5cm diameter, a 200µm mesh
net with a 1L sampling bottle) was also used. It was
deployed from variable depths depending on distinctive
signals of fluorescence and backscatter in the water
column.
WET LAB METHODS
Samples were prepared in the wet lab ready for on-shore
lab analysis.
Phytoplankton
- 50ml of each sample was taken straight from the Niskin
bottle and was added to 1ml Lugols Iodine solution to
preserve phytoplankton.
Oxygen
- 100ml of each water sample was decanted straight from
the Niskin bottle into a glass vial. It was made sure
that the water overflowed to remove any bubbles. 1ml of
manganese chloride and then 1ml of alkali-iodide were
added (Winkler, 1888) and the vial was inverted
carefully to mix the solution. Samples were stored in a
temperature controlled container.
Nitrate, Phosphate and Dissolved Oxygen
- samples were filtered using GFF filters (0.7 µm) and
filtering apparatus. 50ml of each sample were stored in
glass (nitrate and phosphate) and plastic (dissolved
silicon) containers, which were kept in a temperature
controlled environment.
Chlorophyll
- the filter papers used to filter the nitrate,
phosphate and dissolved silicon samples were placed in
6ml of 90% acetone and stored in the fridge.
ON SHORE LAB METHODS
CHEMICAL METHODS
Dissolved Silicon–
the dissolved silicon analysis was done using a slightly
modified method from Mullin & Riley (1955). Using a 5ml
hand pipette, 5ml of sample was added to each tube –
which then had 2ml molybdate solution added and left for
10 minutes. 3ml of mixed reducing agent was added to all
samples, standards and blanks, and then allowed to stand
for 2 hours. The mixed reducing agent (MMR) consisted of
10ml metol sulphite, 6ml oxalic acid, 6ml sulphuric acid
and 8ml MQ water. Once 2 hours had passed the absorbance
of dissolved silicon was measured using a U-1800
Spectrometer set at wavelength of 810nm – the samples
were individually added to a 4cm cell which was cleaned
before measurements. Construction of a calibration curve
from the results obtained can be used to determine the
dissolved silicon concentration of each unknown sample.
Phosphate–
the methods of Parsons T. R. Maita Y . and Lalli C.
(1984) were followed to determine phosphate
concentrations.
Dissolved Oxygen
– the dissolved oxygen concentration was determined
using the method of Grasshoff, K., K. Kremling, and M.
Ehrhardt (1999).
Nitrate
– concentration was determined using the ‘nitrate by
flow injection analysis’ method of Johnson K. and Petty
R.L. (1983)
BIOLOGICAL METHODS
Zooplankton
- Upon collection using the plankton net, organisms were
preserved in diluted formalin. On-shore, zooplankton
abundance and species information was obtained by
analysing 10ml subsamples in a Bogorov tray under a
light microscope.
Phytoplankton-
The number of phytoplankton cells per m3 was found by
placing 1ml of concentrated sample into a Sedgewick-Rafter
chamber. Species were identified, counted using a light
microscope and then the resulting number was adjusted
for concentration and quantity to find the abundance per
m3.
Chlorophyll
- the chlorophyll concentration of each sample was
found as stated by Parsons T. R. Maita Y . and Lalli C.
(1984).
OFFSHORE BOAT PRACTICAL-
Bio -
Chemical -
Physical -
Summary -
Back to top
INTRODUCTION
How do vertical mixing processes in the waters off
Falmouth affect, directly and indirectly, the structure
and functional properties of plankton communities?
The usual trend expected of offshore waters is; a
decline in temperature and an increase in salinity with
depth, and, a peak in plankton abundance at the
thermocline. We predict depletion in nutrients and a
decline in the oxygen content of the water where
plankton is abundant. By obtaining chemical, physical
and biological data we aim to explain the distribution
of plankton communities and compare this to expected
trends
OFFSHORE METHOD
FIELD METHODS
The initial plan for the morning of 27th June 2012 was
to follow a transect starting at Black Rock (50⁰08.68N
005⁰01.74W) bearing due south towards Manacles Point
(see Figure 2.1). CTD profiles and water samples were to
be obtained at 4 sampling stations; Black Rock, Manacles
Point, and at two stations between these locations. Upon
sampling at Black Rock, heavy fog conditions were
observed, deeming the remainder of the planned transect
unsafe to execute. Samples were collected at this
station along with CTD, fluorometer and ADCP data
however; the plankton net was not deployed.
Consequently, a revised plan to carry out time-series
measurements at a single location in the harbour was
adopted. The location of the time-series station
(Station 2) deviated about 50⁰09.543N 005⁰04.012W due to
the drifting of the vessel (see Figure 2.1). CTD and
fluorometer data was collected every half hour starting
at 0845 and, every hour water samples were taken at
depths of interest. The plankton net was also deployed
every hour. See “Field Methods” for more detail.

Figure 2.1
Date:
27/06/2012 (day before neap tide)
| |
LW |
HW |
LW |
HW |
| Time
(UTC) |
04:27 |
10:20 |
16:51 |
22:47 |
|
Tidal height (m) |
1.2 |
4.4 |
1.3 |
4.6 |
Table 2.1:
|
Station |
Latitude N |
Longitude W |
Time
(UTC) |
Depth (m) |
Weather |
| 1
(black rock) |
50°
08.735 |
005°
01.516 |
07:40 |
31.88 |
Fog, calm |
| 2
(time series) |
50°
09.514 |
005°
04.012 |
08:45 |
8.28 |
light rain, fog |
| 2
(time series) |
50°
09.543 |
005°
04.012 |
09:15 |
8.40 |
fog |
| 2
(time series) |
50°
09.541 |
005°
04.013 |
09:45 |
8.55 |
light rain, fog |
| 2
(time series) |
50°
09.541 |
005°
04.012 |
10:15 |
8.72 |
less fog, calm |
| 2
(time series) |
50°
09.541 |
005°
04.011 |
10:45 |
8.61 |
fog |
| 2
(time series) |
50°
09.539 |
005°
04.015 |
11:15 |
8.93 |
less fog, calm |
LAB METHODS
Samples were prepared in the wet lab ready for on-shore
lab analysis as stated in the “Wet Lab Methods”
Physical
- CTD data was managed and graphed using ‘Sigmaplot’.
ADCP data was displayed using Winriver.
Chemical
- Samples collected were analysed to quantify dissolved
silicon, dissolved oxygen, chlorophyll and, nitrate and
phosphate. Methods of analysis are stated in “Lab
Methods”.
Biological
- Both zooplankton and phytoplankton species were
identified and counted. The exact method is stated in
“Lab Methods”.
BLACK ROCK (STATION 1)
RESULTS
PHYSICAL
ADCP
Velocity appears to vary little with depth at station 1
(fig. 3.3), there is an area of greater velocity in the
far west of the plot, this rapid increase in velocity
with maximum velocity of ~ 1ms-1, this patch of rapidly
increased velocity may be an anomaly as it is
significantly greater than the water surrounding it.
Although it may also be related to the rapid change in
topography of the sea bed at the same location.
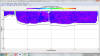
Figure 3.3: ADCP profile showing flow
rate (m/s) from Station 1 (Black Rock) at 0745 UTC
CTD
Figure 3.2 Illustrates the CTD data obtained at the
first station, Black Rock. The surface temperature
recorded was 13.40⁰C which remained constant until
around 2.02m depth before rapidly decreasing between
depth 2.5m and 3.80m to a temperature of 12.93⁰C. The
temperature then decreases slowly, and remains constant
at 12.75⁰C until a depth of 23.59m. Temperature
decreased to 12.58⁰C by the end of the profile at 26.92m
depth. The fluorescence data on the CTD fluctuated
greatly with depth but generally remained within the
range of 0.11 and 0.15V with a few exceptions such as at
the surface of 0.10V and peaks of increased fluorescence
at 12.82m a value of 0.17V. Transmission was used as an
indication of turbidity in the water column and was
lowest at the surface, and increased rapidly within the
first 0.52m depth of the water column and then remained
at a constant value around 3.89% until 27m. The salinity
did vary significantly with depth, at 0.28m the lowest
recorded salinity was 34.10 which increased to 35.1 at
3.85m. After this, salinity remained constant as depth
increased, with an exception of a slight increase to
35.23 at 25.95m depth.

Figure 3.2: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 1 (Black Rock)
at 0745 UTC
LIGHT
|
Equation of
Regression Line |
Gradient of
Regression Line *-1 |
Equation to
Calculate Ksensor |
Ksensor
(m-1) |
Secchi Depth (m) |
Equation to
calculate KSecchi
|
KSecchi
(m-1) |
KSecchi
-Ksensor |
|
y=24.217-4.920x
|
4.920 |
1/(4.920) |
0.203 |
5 |
1.44/5 |
0.29 |
0.087 |
Table 3.1 Station 1 light attenuation information. The
equation of the regression line and negative reciprocal
of the gradient of the line. From this gradient Ksensor
is calculated. Secchi depth is recorded in meters. This
sechi depth is used to calculate a second light
attenuation coefficient (KSecchi). The
final column shows the range between these two light
coefficient values.
Station 1 has a surface light irradiance of ~375m-2,
(Figure 3.5) as depth increases light irradiance
decreases exponentially, a significant decrease in
irradiance is shown over the surface 5m, with light
irradiance values of ~40Wm-2 at 5m depth. 1% of surface
light irradiance is found at ~17.5m depth. The natural
log of light irradiance against depth (Figure 3.6)
follows a relatively straight line between depths of ~3m
and the maximum depth of the profile, the top 3m of the
water column show a greater Ln(Ez) value than expected.
The equation of the regression line shown in figure P,
is shown in table T. Using the negative reciprocal of
the gradient of the regression line the attenuation
coefficient can be calculated (Ksensor ). The
attenuation coefficient calculated from the Secchi depth
(Ksecchi) is also shown in table T. These
two different attenuation coefficients appear to show a
significant difference, with Ksecchi being
~40% greater than Ksensor

Figure 3.5: light irradiance curve from CTD data
measured in Wm-2 at Station 1 (Black Rock) at
07.45 UTC

Figure 3.6: natural log of light irradiance(ln(Ez)) with
depth from CTD data at Station 1 (Black Rock) at 07.45
UTC
Table T Station 1 light attenuation information. The
equation of the regression line and negative reciprocal
of the gradient of the line. From this gradient Ksensor
is calculated. Secchi depth is recorded in meters. This
sechi depth is used to calculate a second light
attenuation coefficient (KSecchi). The final column
shows the range between these two light coefficient
values.
CHEMICAL
The Station 1 chemical data was recorded from two
depths. The Oxygen saturation profile decreased from
107% saturated at 2m to 99% saturated at a depth of 10m
.Dissolved silicon levels also decreased with depth, a
concentration of 1.02 µmol/l at a depth of 2m was
recorded. This then decreased to 0.79 µmol/l at 10m.
Phosphate levels showed an increase from 0.037 µmol/l of
phosphate recorded at 2m to 0.067 µmol/l at 10m.
BIOLOGICAL
Figure 3.4: Shows a larger abundance of phytoplankton at
2m than 10m. Guinardia flaccida, Rhizosolenia alata and
Rhizosolenia delicatula are the most abundant species
present in the water column at this time. With cell
count estimates of 1.2 x 107, 1.8 x 107 and 1 x 107 per
m3 respectively. Species diversity is low at this time.

Figure 3.4: phytoplankton cell count from water samples
taken at 2 and 10m depth measured in cells per m3
at Station 1 (Black Rock) at 0745 UTC
Figure 3.7 illustrates that the highest chlorophyll
measurements taken at both a shallow depth of 2m and the
deepest depth of 10m were taken at the first station at
Black Rock.

Figure 3.7 Chlorophyll (µg/l) measured in the lab by
Fluorometer from Niskin bottle samples at Station 1
(Black Rock) at 07.45 UTC
DISCUSSION
The weak thermocline and strong halocline indicates a
shallow cold less saline surface layer of the water
column. These physical gradients occur within the first
5m. In the Western English channel a weak seasonal
thermocline occurs due to deep waters and relatively low
tidal mixing (Mathews, 1911) This is supported by the
Richardson number data, which shows laminar flows in the
surface 10m. The halocline is formed by output of less
saline so therefore less dense water from the River Fal.
This is more buoyant than the cooler, more saline waters
of the Western English Channel and creates a strong
salinity gradient.
The 2m sample collected water from above the apparent
thermo and haloclines, the 10m sample retrieved from
below. The shallow water sample indicated low
phosphate, high dissolved silicon levels. The CTD
fluorescence data (Figure 3.7) depicts the surface
waters to be relatively low in chlorophyll compared to
data collected at depth. However phytoplankton cell
counts taken at 2m and 10m illustrates phytoplankton
abundance to be greater in the surface water than
further down the water column. The chlorophyll
concentration calculated from the water samples, support
the fluorescence data collected by the CTD, as it shows
a greater concentration at 10m than 2m. This supports
the theory that phytoplankton growth becomes limited by
the depletion of nutrients (Hooligan & Harbour, 1977).
Figure 3.7 illustrates that the
highest chlorophyll measurements taken at both a shallow
depth of 2m and the deepest depth of 10m were taken at
the first station at Black Rock.
TIME SERIES (STATION 2)
PHYSICAL RESULTS
ADCP
The ADCP data at Station 2 (Figure 4.1) shows velocities
ranged between 0.001 m/s and 0.250 m/s throughout the
times series. The layer of water between 2.59m and 3.00m
exhibits faster flow velocities of up to around 0.400m/s
throughout the time series.

Figure 4.1: ADCP flow
rate profiles (m/s) taken between 0815 and 1115 every
half hour at Station 2
RICHARDSON NUMBER
The Richardson number is a dimensionless number used to
evaluate the mixing conditions in the water column.
The Richardson number was calculated using averages over
the course of every half hour at Station 2 and once at
Station 1.
At 0845 the Richardson number remained under 0.2500 for
most of the water column with the only exceptions at
2.59m (Ri=0.2787) and 6.59m (Ri=0.4348). This was also
true for the water column at 0915 with the only
exception being 0.3032 at 2.59m. At 0945, Ri increased
with depth beginning at 0.0404 at 2.59m and increasing
to 0.7860 at 6.59m, however, Ri did not surpass 1.0000.
At 1015, the Richardson number remained above 0.2500 for
the entire water column apart from at 2.59m and 4.59m
where Ri was 0.1359 and 0.1057 respectively. The
Richardson number at 1045 was lowest at 3.59m at a value
of 0.0461 and the maximum Ri was 13.9767 at 6.59m. At
1045, Ri increased with depth. At 1115, Ri was over
1.0000 at depths of 3.59m and shallower however, this
rose rapidly to 3881.3185 at 4.59m and then proceeded to
drop again to 0.6661 at 5.59m and then again to 0.0592
at 6.59m (Figure 4.2).

Figure 4.2: Time series
of Richardson numbers (Ri) taken between 0830 and 1120
calculated using ADCP data at Station 2
CTD
Note: in order to gain accurate
results with the CTD profiler, it was necessary to lower
the sampler to just below the surface (commonly around
1.00m depth) for a few minutes, allowing it adjust to
the condition of the water column. It is for this reason
that there may be horizontal 'lines' of data in places,
where the CTD was adjusting. Transmission values are
also high upon entering the water which is a result of
the instrument adjusting to the transition from the air
to the water.
A 1.25m thick fresher surface water layer was evident in
Figure 4.3, with a salinity of 34.00. Below this depth
was a halocline, extending to 2.45m depth with salinity
increasing to 34.35. Below the halocline, salinity
increased gradually to 34.55 by 6.79m depth. Another
sharp increase in salinity was noted at the bottom of
the profile, increasing to 34.82 by 7.59m depth. The
temperature data also indicated a different surface
layer 1.25m thick with a temperature of 14.2⁰C. Below
this layer a thermocline was evident, extending to a
depth of 2.60m and decreasing to 13.91⁰C. The
temperature stayed fairly stable for the remaining
depths; however, it decreased rapidly from 13.79⁰C to
13.42⁰C between depths of 6.79m and 7.64m. Transmission
increased to 3.45 by 0.55m depth, and remained between
3.50%-3.60% as depth increased. Fluorescence fluctuated
greatly throughout the water column but remained within
a range of 0.09V and 0.11V, with the exception of the
large peaks at the depths 0.87 m, 7.31m and 7.68 m,
which reached values of 0.16V.
Figure 4.4 shows the physical parameters of Station 2 at
0915. A surface layer of water 1.75m thick had a
temperature of around 14.15⁰C. By 2.2m depth temperature
had rapidly decreased to 13.95⁰C, representing a
thermocline. Below this depth, temperature remained
fairly constant, until a sharp decrease from 13.83 to
13.55⁰C between 6.38m and 7.36m depth. The surface layer
was also characterised by a lower salinity than the rest
of the water column, of around 34.00 in the top 1.75m.
Below this depth, salinity gradually increased to 34.73
at 7.36m depth. Fluorescence fluctuated from 0.07V to
0.12V throughout the water column. Transmission
increased to 3.58% by a depth of 0.60m, and beyond this
depth remained constant
.
Figure 4.4: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 2 at 0915
Data acquired at 0945 displayed in Figure 4.5 appeared
to show no distinctive surface layer as seen in previous
time-series profiles. Salinity increased fairly steadily
from 33.69 to 34.81 over the 7.78m profile. Temperature
also decreased steadily over this depth, from 14.47⁰C to
13.43⁰C. After an initial increase in the top 0.50m of
the water column, transmission remained close to 3.61%
throughout the whole profile. Fluorescence fluctuated
between the values 0.08V and 0.12V with the lowest value
at the surface of 0.08V and a peak at 7.88m depth with a
value of 0.13V.

Figure 4.5: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 2 at 0945
The time-series profile of Figure 4.6 (recorded at 1015)
appeared to show a distinct surface layer, despite this
stratification not being present in the profile acquired
30 minutes prior (Figure 5.5). Salinity data suggested
a surface layer thickness of 2.04 m, with a salinity of
34.02. Below this depth, salinity increased to 34.81m at
7.45m. Interestingly, below this depth until 7.78m
salinity decreased to 34.72. Temperature showed an
inverted distribution to salinity, with a 1.98m thick
surface layer of 14.19⁰C. Below this depth, until 7.45m,
temperature decreased gradually to 13.44⁰C, before
increasing to 13.56⁰C by 7.78m. Again, fluorescence
greatly fluctuated with depth, remaining within the
range of 0.09V and 0.11V. The greatest peak occurred at
a depth of 4.53m with a value of 0.13V. Transmission
increased to a value of 3.52% by 0.49m depth, then
remained fairly constant throughout the profile with the
exception of a slight decrease at 7.78m.

Figure 4.6: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 2 at 1015
In Figure 4.7 temperature decreased steadily from
14.30⁰C to 13.44⁰C over the entire 7.88m of the profile.
Salinity increased steadily with depth throughout the
profile from 33.98 to 34.81. Transmission increased to
3.50% in the top 0.69m, and remained close to 3.60-3.70%
for the remainder of the profile. Fluorescence
fluctuated within the range of 0.09V and 0.13V with the
smallest fluorescence value of 0.07V recorded at the
surface and the greatest at 7.95m depth of 0.16V.

Figure 4.7: CTD
data measuring temperature (⁰C), fluorescence (V),
salinity and transmission (%) measured at Station 2 at
1045
Figure 4.8, showing a profile taken at 1115, illustrates
that temperature decreased from 14.43⁰C at the surface
to 13.42⁰C at 8.62m depth. Salinity increased from 33.95
to 34.83 from the surface to 8.62m depth. Transmission
increased to 3.56% rapidly within the top 0.88 m, and
remained constantly near this value for the remainder of
the profile. Fluorescence values fluctuated from 0.07V
to 0.13V throughout the whole profile.

Figure 4.8: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 2 at 1115
The greatest surface irradiance was found at times 0945
and 1015 (Figure 4.9). It is also appears that very
little light was absorbed in the surface ~30cm of the
depth profiles, as here the gradient of the light
irradiance curve is near vertical. This could be due to
the sensor being located at the highest point on the
rosette sampler, and the depth reading being taken from
the bottom of the sampler. At most depths the natural
log of Ez followed a near straight line (Figure 4.10).
In surface waters the linear relationship was altered,
which could again be due to the location of the CTD on
the rosette sampler.

Figure 4.9: time series
of light intensity (Ez) measured using a fluorometer at
Station 2 between 0845 and 1115
The attenuation coefficients calculated from the light
sensor on the CTD (Ksensor) showed a greatest value of
0.461m-1 at 1015, and a lowest value of
0.334m-1 at 0845. The range between these is
equal to 0.127m-1, which is over 25% of the
value at 1015. This showed a significant change between
stations, however there appeared to be little
relationship with time. The three attenuation
coefficients calculated with the Secchi disk depths
showed a maximum value of 0.36m-1 at 0845,
and a minimum value of 0.29m-1 at 1045. The
range here was also significant as it was equal to ~20%
of the KSecchi value at 0845. There appeared to be an
inverse relationship, as the attenuation coefficient
decreased as time increased.
|
Time
(UTC) |
Equation of
Regression line |
Gradient of
Regression Line *-1 |
Equation to calc
Ksensor |
Ksensor
(m-1) |
Secchi depth (m) |
Equation to calc
KSecchi
|
KSecchi
(m-1) |
KSecchi
-Ksensor |
|
0845 |
y=15.823-2.998x |
2.998 |
1/(2.998) |
0.334 |
4 |
1.44/4 |
0.36 |
0.026 |
|
0915 |
y=16.462-2.186x |
2.186 |
1/(2.186) |
0.457 |
No data |
n/a |
n/a |
n/a |
|
0945 |
y=17.378-2.917x |
2.917 |
1/(2.917) |
0.343 |
4.5 |
1.44/4.5 |
0.32 |
-0.023 |
|
1015 |
y=16.462-2.186x |
2.186 |
1/(2.186) |
0.461 |
No data |
n/a |
n/a |
n/a |
|
1045 |
y=17.378-2.917x |
2.917 |
1/(2.917) |
0.343 |
5 |
1.44/5 |
0.29 |
-0.053 |
|
1115 |
y=16.126-2.633x |
2.633 |
1/(2.633) |
0.379 |
No data |
n/a |
n/a |
n/a |
Table 4.1: Includes the equation of each regression line
shown in Figure 4.10, the gradient of these lines
multiplied by -1, The equation used to calculate Ksensor,
as well as the Ksensor value. Also shown is the Secchi
disk depth for those times where a Secchi depth had been
recorded. This is then used to calculate another
attenuation coefficient KSecchi, this is done by
dividing 1.44 by the secchi depth (m). The final column
shows the range between the two attenuation coefficients
calculated

Figure 4.1: ADCP flow
rate profiles (m/s) taken between 0815 and 1115 every
half hour at Station 2
CHEMICAL RESULTS
At 0845 dissolved oxygen decreased from 109% saturation
at a depth of 0.91m to 105% saturation at 6.94m.
Dissolved silicon levels also declined from 2.96 µmol/l
at 0.91 to 2.18µmol/l at 6.94m. Phosphate levels
decreased from 0.049 µmol/l to 0.037µmol/l over the same
depth range (Figure 5.1).

Figure 5.1: Phospohate
(µmol/L), Silicon (µmol/L) and 02 (% saturation)
profiles at Station 2 at 0845
At 0945, dissolved oxygen dropped from 110% saturation
to 67% over 7.32m, with the steepest drop from 107% at
4.27m to 67% at 7.32m. Dissolved silicon levels
increased from 2.18µmol/l at 0.93m to 2.29 µmol/l at
4.27m and then 3.26 µmol/l at 7.32m. Phosphate
concentrations increased from 0.037 µmol/l at 0.93m to
0.073µmol/l at 4.27m, and then decreased slightly to
0.061 µmol/l at a depth of 7.32m (Figure 5.2).

Figure 5.2: Phospohate
(µmol/L), Silicon (µmol/L) and 02 (% saturation)
profiles at Station 2 at 0845
At 1045, the oxygen concentration rises from 78% at
0.77m depth to 79% at 4.23m. It then declined slightly
to 77% at 6.86m. Dissolved silicon levels decreased from
2.80µmol/l at 0.77m to 0.54µmol/l at 4.23m, then
increased slightly to 1.76µmol/l at 6.86m. Phosphate
levels at 1045 Station 2 followed a similar pattern to
oxygen concentration with a slight increase of
0.055µmol/l at 0.77m to 0.073µmol/l at 4.23m, then
decreasing to 0.067µmol/l at 6.86m (Figure 5.3).

Figure 5.3: Phospohate
(µmol/L), Silicon (µmol/L) and 02 (% saturation)
profiles at Station 2 at 10.45
BIOLOGICAL RESULTS
Figure 6.1 shows there are 2 groups which were dominant
throughout the time series, Copepoda and Copepoda
Nauplii, with Copepoda numbers varying from 1191 cells/m3
to 4546 cells/m3 whilst Copepoda Nauplii
numbers varied from 2490 cells/m3 to 7144
cells/m3. It can be seen that the majority of
groups increased in number from 0845 to 1045; and groups
that increased over the time series included Copepoda,
Copepoda Nauplii, Decapoda Larvae, Cirripedia Larvae,
Hydromedusae and Appendicularia. Groups that were not
present at the start of the time series but were by the
end include Ctenophora and Echinoderm larvae.

Figure 6.1: Zooplankton
cell counts collected by vertical trawl measured in
cells/m3 for 0845,0945 and 1045 for station 2
Figure 6.2 showed a larger abundance of phytoplankton at
7.00m than 1.00m. A greater number of species were seen
to be present in deeper waters. Rhizosolenia alata,
Rhizosolenia stegera, Rhizosolenia stolterfothii and
Eucampala spp. were all observed at 7.00m but
not 1.00m. Rhizosolenia setigera was the most
abundant species with cell counts of 14 cells/mL.

Figure 6.2:
Phytoplankton cell counts in cells per ml at 1 and 7m
sampled at Station 2 at 0845
Figure 6.3 showed the greatest abundance of
phytoplankton in the water column at 1.00m. Species
diversity at 0945 was high. Guinardia flaccida,
Thalassiosira spp. and Rhizosolenia
delicatula were the most abundant species in the
water column with more than 13 cells/mL. Guinardia
flaccida and Rhizosolenia imbricata were
present at all depths in the water column.

Figure 6.3:
Phytoplankton cell counts in cells per ml at 1, 4.5 and
7m sampled at Station 2 at 0945
Figure 6.4 showed greatest abundance of phytoplankton at
depths of 7.00m. Species variety was large across all
depths of the water column. Guinardia flaccida
was the most abundant species with over 45 cells/mL, and
this species was present at all depths of the water
column. At 7.00m there were three species with large
observed cell counts, Alexandrium spp., Guinardia
flaccida and Rhizosolenia alata.

Figure 6.4: time series
of chlorophyll (µg/l) calulcate in the lab at station 2
between 0845 and 1045
The chlorophyll samples at Station 2 that were taken
around 0.90m had a higher chlorophyll level than at
7.00m, but when samples were taken at intermediate
depths such as at 0945 and 1045 there appeared to be a
non-linear relationship with depth. At 0945 there was a
chlorophyll maximum with a value of 2.27 µg/l at 4.30m,
whereas at 1045 there was a chlorophyll minimum of
1.65µg/l at 4.20m. At Station 2 there were fluctuations
in chlorophyll concentration throughout the time series
which appeared to exhibit no clear trend (Figure 6.5).
DISCUSSION
The tide was flooding the harbour for almost the
entirety of the sampling period (high tide was at 1020,
Table 2.1). This explains the layer of fresher water
present in the top 1.20m until 0915. Because the tide
was still flooding the estuary, freshwater still
dominated the top layer and maintained a stratified
system in the harbour. This was further supported by the
temperature gradient at 0845 that exhibited a sharp drop
at 1.30m, at almost exactly the same location as the
halocline. As the morning progressed and more saline
water entered the harbour, the stratification created by
differing densities of fresh and salt water was broken
down. This can be seen from 0945 until 1145 where
temperature decreased steadily rather than exhibiting a
sharp drop at 1.20m as seen at previous times. Even
though high tide occurred at 1020, the direction of
tidal currents had yet to change for sampling points
1045 onwards due a delay in the tidal height between the
mouth of the estuary and the harbour. In all of the
profiles there was an increase in salinity with depth
and a decrease in temperature with depth. This is
expected because the more saline, colder water flowing
in from offshore is denser than the fresh-water inputs.
The breakdown of stratification over the course of the
sampling period was further supported by the general
increase in Ri number. As the morning progressed, the
number of points in the water column where Richardson
number exceeded 1.0000 increased. This indicates that
the water column is unstable and encourages mixing of
previously stratified layers. This was further
supported by the chlorophyll maximum present at 4.20m at
0945 (phytoplankton inhabited the waters just below the
thermocline) whereas at 1045, there was a chlorophyll
minimum at around the same depth. This shows that the
stratification is beginning to break down and it is no
longer beneficial for the plankton to concentrate
themselves at this particular depth.
There was no particular trend spatially or temporally
with respect to chlorophyll; this is consistent with our
transmission and fluorescence data and is due to enough
light and nutrients being available over the entire
water column for the phytoplankton to use. However, in
order to make a definitive conclusion, more data must be
acquired. It can be assumed that any variation in
transmission, chlorophyll and fluorescence data was due
to small scale temporal and spatial changes in currents
and nutrient availability.
The harbour in which we conducted the time series has a
flushing time of around 2.4hours (IST and Falmouth
Harbour Commissions, 2012). This leads to extremely
regular replenishment of nutrients to support high
levels of phytoplankton. By looking at our data, a
decline in the concentrations of nutrients and dissolved
oxygen with depth is evident, indicating an uptake of
these nutrients by plankton deeper in the water column.
The overall number of zooplankton species increased over
the course of the day. This is due to more nutrient
rich, salt water flooding the harbour and because light
levels increase as we approach noon. This is supported
by a depletion of oxygen and nutrients over the course
of the day as numbers of respiring plankton rise. Both
light levels and high nutrient concentrations in the
water column contribute to more phytoplankton and
therefore, more for the zooplankton to consume.
DISCUSSION OF OFFSHORE
PRACTICAL
ESTUARINE PRACTICAL
- Bio -
Physical -
Chemical -
Summary
- Back to top
INTRODUCTION
How does the estuary
behave as a transition zone between the freshwater
riverine and ocean environments?
Due to the enclosed nature of estuaries, their
properties with respect to nutrient concentrations,
oxygen availability, plankton abundance and light
attenuation sometimes vary wildly. They tend to differ
from the conditions found in both saline and riverine
environments and do so in a graded manner over short
spatial scales. Furthermore, they are often home to
abnormal build-ups of pollutants. Our objective is to
sample over a range of salinities whilst travelling down
the estuary in order to observe these changes and make
conclusions based on the results collected.
FIELD METHODS
Fieldwork was carried out on the 3rd of July 2012,
aboard the RV Bill Conway. Figure 7.1 shows a map of
sampling stations and routes taken on the day, and exact
positions are recorded in table 7.1. Times of high and
low tides are displayed in table 7.2, along with local
weather information and depths of the water column. At
each station, CTD profiles and water samples at
different depths were taken, ADCP transects across the
estuary at each station were also recorded. When leaving
Stations 2, 5 and 7, zooplankton trawls (using a 45.8cm
diameter net) were carried out for 5 minutes and the
volume of water flowing through the net was measured by
a revolving flow meter. The CTD available did not have
Niskin bottles attached therefore; water samples could
only be taken using a hydroline and messenger system.
This meant that samples were limited to 9m depth.
Sampling was limited by the number of lugols bottles
(for phytoplankton) and oxygen bottles we had available
as there were only 5 bottles filled with lugols iodine
available, and so decided to use two at Station 2, two
at Station 4, and one at Station 7. There were sixteen
oxygen bottles available, out of which five were used at
Station 5 as this was a specific site of interest.
Underway water samples (pumped on board from the surface
of the water column) were collected at increasing
salinity values whilst travelling between stations with
the intention to measure dissolved silicon, chlorophyll
and nitrate concentrations later on in the lab. The
temperature, salinity, latitude and longitude were also
recorded for each corresponding underway sample.
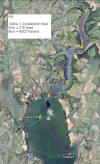
Figure 7.1: map of
sampling stations and transects of the estuarine survey
taken on 03/07/2012
Date: 3/07/2012
| |
HW |
LW |
HW |
LW |
|
Time (UTC) |
0425 |
1110 |
1648 |
2340 |
|
Tidal height (m) |
4.9 |
0.6 |
5.2 |
0.5 |
Figure 7.2
|
Station |
Latitude |
Longitude |
Time |
Depth (m) |
Weather |
|
1 |
50
̊ 10.048N |
005
̊02.491W |
0814 |
29.8 |
light rain |
|
2 |
50
̊ 14.416N |
005
̊00.869W |
0908 |
5.0 |
light rain |
|
3 |
50
̊ 13.330N |
005
̊01.610W |
1005 |
12.5 |
light rain |
|
4 |
50
̊ 12.546N |
005
̊01.649W |
1050 |
4.2 |
light rain |
|
5 |
50
̊ 12.180N |
005
̊02.434W |
1115 |
16.2 |
light rain |
|
6 |
50
̊ 11.623N |
005
̊02.802W |
1210 |
16.0 |
light rain |
|
7 |
50
̊ 10.672N |
005
̊01.553W |
1242 |
27.8 |
light rain |
LAB METHODS
Samples were prepared in the wet lab ready for on-shore
lab analysis as stated in the “Wet Lab Methods”
Physical - CTD data was managed and graphed using
‘Sigmaplot’. All Salinity data taken from the CTD had to
be multiplied by 0.95 due to calibration issues. ADCP
data was displayed using Winriver.
Chemical - Samples collected were analysed to
quantify dissolved silicon, dissolved oxygen,
chlorophyll and, nitrate and phosphate. Methods of
analysis are stated in “Lab Methods”.
Biological - Both zooplankton and phytoplankton
species were identified and counted. The exact method is
stated in “Lab Methods”.
PHYSICAL RESULTS OF ESTUARINE PRACTICAL
ADCP
STATION 2
Maximum velocities were reached in the surface waters
above 1.89m (Figure 8.1), with a maximum velocity of
0.362ms-1 in the far west of the estuary.
Velocities decreased with depth, minimum velocity was at
2.89m at 0.021 ms-1. From the stick ship
track, the dominant flow observed was south westerly,
with a maximum velocity of 0.275 ms-1(Figure
8.2).

Figure 8.1 ADCP flow
rate profiles (m/s) for station 2 taken at 0908

Figure 8.2: ADCP ship
track for station 2 taken at 0908
STATION 3
The maximum flow velocity was found in the surface
waters at the centre of the channel at 0.398 ms-1(Figure
8.3). In the centre of the channel, velocity of the
surface waters above 5m was greater than velocity of the
water below 5m. The minimum velocity recorded was 0.004
ms-1 at 7.39m. The surface flow either side
of the main channel was considerably lower than the
surface flow in the channel. The shallow waters to the
west of the velocity plot, flow was low with no
velocities recorded above 0.200 ms-1. The
velocities on the eastern side of the transect regularly
reached velocities of over 0.200 ms-1 with a maximum
velocity of 0.246 ms-1. The stick ship track
plot indicated weak southerly flows with no velocities
over 0.15ms-1(Figure 8.4).

Figure 8.3 ADCP flow
rate profiles (m/s) taken for Station 3 at 1009

Figure 8.4: ADCP ship
track for Station 3 taken at 1009
STATION 4
The maximum flow velocities were found within the centre
of the channel up to 0.304 ms-1 (Figure 8.5).
Waters either side of the channel were found to have
lower velocities. Generally, velocities increased
towards the centre of the plot. Velocities did not vary
with depth. The stick ship track plot showed an easterly
flow direction with a maximum velocity of 0.124 ms-1
(Figure 8.6).

Figure 8.5: ADCP flow
rate profiles (m/s) at Station 4 at 1054

Figure 8.6: ADCP ship
track for Station 4 at 1054
STATION 5
Low velocities were consistently observed throughout the
water column. The eastern side of the transect showed
slightly greater velocities reaching up to 0.447 ms-1
at 1.39m(Figure 8.7). However, the far west of the
transect showed a maximum velocity of 0.250 ms-1.The
centre of the channel showed no significant variation
with depth. However, the four largets velocities (0.24
ms-1 ,0.241 ms-1 ,0.268 ms-1
,0.248 ms-1) were found between 4.89m and
9.89m. From the stick ship track plot a northerly flow
was observed, with greatest velocities towards the east
of the channel which reached 0.447 ms-1
(Figure 8.8). Some eddying was present in the far west
of the channel, with a velocity of 0.121 ms-1
flowing southwards.

Figure 8.7: ADCP flow
rate profiles (m/s) taken at Station 5 at 1119

Figure 8.8 ADCP ship
track for Station 5 at 1119
STATION 6
In the surface waters the velocities were greater either
side of the channel than the centre of the channel.
Within the centre of the channel, below a depth of
6.89m, a large area of high velocities was seen to
extend down to the bottom of the water column with a
maximum velocity of 0.47 ms-1 (Figure 8.9).
In water shallower than 6.89m, in the centre of the
channel, the greatest velocity recorded was 0.25 ms-1.
The flow in the centre of the channel was slow and
northerly (Figure 8.10).

Figure 8.9: ADCP flow
rate profiles (m/s) for Station 6 at 1212

Figure 8.10 ADCP ship
track for Station 6 at 1212
STATION 7
Figure 8.11: Richardson
numbers for estuary stations, red line where Ri=0.25,
a=station 2, b=3, c=4, d=5, e=6
In the top 6m at station 3 (Figure 8.11b), Richardson
number readily fluctuated above and below 0.2500.
However at depths greater than 6m, Richardson number was
only lower than 0.2500 at one depth, 8.89m.
Richardson number at station 4 (Figure 8.11c) was
greater than 0.2500 at the majority of depths.
Station 5 (Figure 8.11d) shows a region of the water
column, between 2.89m and 11.89m where Richardson number
is predominantly below 0.2500. However, Richardson
number was above 0.2500 between 3.89m to 4.39m and
,6.89m to 7.39m. Below 11.89m, Ri was lower than 0.25 at
two depths, 12.89m and 14.39m.
The station 6 depth profile (Figure 8.11) showed 3 main
regions where Ri was lower than 0.25. The first of these
was the surface waters down to a depth of 3.39m, here
the Ri was greater than 0.2500 for only a small period
at 2.39m. The second of these regions was from 5.89m to
7.89m, where Richardson number was consistently lower
than 0.2500. Richardson number increased above 0.2500
until 9.39m where it remained below 0.2500 for a further
meter. The final region where Ri was lower than 0.2500,
was from 11.89m to the maximum depth, where Ri was
permanently below 0.25.
CTD
Figure 8.12 shows CTD data for station 1. The
temperature was 14.18oC at an uppermost depth
of 0.40m. This decreased steadily to 13.04oC
by 5.34m depth. The temperature remained constant from
5.34m to 8.12m at around 13.02oC, before it
decreased to 12.80oC by 11.26m, and remained
near this value for the rest of the water column.
Salinity increased steadily from 33.77 at the surface to
34.97 at 5.34m depth. The salinity remained at 34.97
until 8.39m depth. By 9.45m, salinity increased to
35.05, and stayed near this value for the remainder of
the profile. Transmission, whilst fluctuating, increased
from 4.35% at the surface to 4.45% by 11.26m, where it
remained fluctuating around this value with increasing
depth. Fluorescence fluctuated widely throughout the
whole profile, generally about 0.21 mg/m-3,
ranging from 0.15 to 0.43 mg/m-3. The two
biggest peaks occurred at 12.06m and 22.69m, with values
of 0.48 and 0.39 mg/m-3 respectively.

Figure 8.12: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 1
The temperature of the column at station 2 (figure 8.13)
decreased steadily from 15.26oC to 15.11oC
in the surface 2.92m, as shown in figure 2. For the
remainder of the profile to a depth of 3.80m the
temperature remained close to 15.10oC.
Salinity increased from 23.22 to 29.29 from the surface
to 2.60m. Beyond this depth, salinity values remained
fairly constant at 29.4. Fluorescence decreased from
0.42V at the surface to 0.29V at 2.71m depth, and
exhibited fluctuations. Below this depth, values
remained close to 0.29V, with the exception of a peak of
0.35V at 3.15m and 0.40V at 3.80m. As a general trend,
transmission increased from 3.33% to 3.69% from the
surface to 2.23m depth, with the exception of increasing
to 3.39% at a depth of 0.52m. Values remained close to
3.69% for the remainder of the profile.

Figure 8.13: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 2 at 0945
Figure 8.14 shows the CTD profile for station 3. There
was a temperature of 15.05 oC at the surface,
this dropped gradually to around 15.00 oC at
4.03m then decreased steadily from there to around 14.46
oC at 10.56m. Salinity increased steadily as
depth increased starting at 27.97 at the surface and
then rose to 32.44 at 10.56m. Transmission dropped from
around 3.91% at the surface to 3.90% at 0.16m. It then
increases steadily with depth and reached a maximum of
4.10% at 10.10m. Transmission followed the same pattern
as salinity, however, a small fluctuation existed where
rate of transmission rose from 3.94% at 4.03m to 4.01%
at 5.12m. Fluorescence exhibited a decline with
increasing depth. The surface fluorescence was around
0.32V and showed a sharp increase to 0.39V at 1.02m.
This then declined steadily to 0.26V at 6.06 m, it
remained at this approximate voltage for the remainder
of the water column. Sharp peaks were present at 3.47m
and at 6.88m to 0.39V and 0.32V respectively.

Figure 8.14: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 3 at 1020
Figure 8.15, displaying data for Station 4, showed a
general increase in salinity from 21.83 to 29.00 over
the whole 3.11m profile, which fluctuated widely
compared to other profiles. Temperature decreased
steadily from 14.95oC at the surface to 14.88oC
at 1.51m depth. This was followed by a more rapid
decrease in temperature to 14.79oC by 1.73m
depth. The decrease in temperature then slowed, reaching
14.76oC by 2.66m depth, before decreasing
more rapidly again to 14.72oC at 3.10m depth.
Omitting two large peaks in fluorescence, one at the
very surface (at 0.90V) and a peak of 0.42V at 0287m,
fluorescence remained between 0.24V and 0.39V for the
whole profile, decreasing gradually throughout.
Transmission values were between 4.07% and 4.08% for the
top 1.51m of the water column, before increasing rapidly
to 4.12% by 1.62m. Below which, transmission increased
gradually to 4.15% at 3.11m depth with values
fluctuating slightly.

Figure 8.15: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 4 at 1104
Temperature declined steadily with depth from 14.75
oC at the surface to 13.23 oC at 14.24m
at station 5, as shown in Figure 8.16. Salinity, on the
other hand displayed a steady increase with depth from
33.83 at the surface to 34.72 at 14.24m. The rate of
increase in salinity was slightly quicker in the top 2m
of the water column. Fluorescence, showed large
fluctuations throughout the water column, particularly
under 4m where there were peaks of 0.41V, 0.38V and
0.37V at 12.59 m, 8.83m and 8.01m respectively. Larger
peaks in fluorescence occurred deeper, despite these
peaks a general decline in fluorescence with depth was
seen from 0.29V at 0.30m to 0.18V at 14.10 m, it then
exhibited a rise as water depth increased, this increase
was rapid between 0.30m and 2.13m where transmission
rose from 4.14% to 4.20%. A sharp decline in
transmission was seen at 2.96m to 4.12%. It increased
rapidly between 2.96m and 5.02m from 4.12% to 4.27%
where it remained until 9.42m, transmission then
increased gradually to 4.35% at 14.16m.

Figure 8.16: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 5 at 1143
Data for station 6 is displayed in Figure 8.17.
Temperature was 14.39 oC at the surface and
remained relatively constant until 4.07m where there was
a sudden decrease to 14.08 oC at 5.01m. This
then decreased steadily to 13.82 oC at 8.29m
and then rapidly to 13.01oC at 11.50m.
Temperature remained at this approximate level as depth
increased. Salinity increased with depth. It remained at
around 32.87 until 2.62m then rose to 33.85 at 8.29m. A
rapid increase to 35.00 at 11.49m then occurred, and
remained relatively constant for the rest of the water
column. Fluorescence fluctuated greatly between around
0.15V and 0.30V, however, a slight decline with depth
was present with fluorescences of 0.23V at 0.21m and
0.19V at 13.98m. Transmission followed the same pattern
as salinity with two rapid increases in % transmission
at around 5m and 10.5m from 4.21% at 3.47m to 4.26% at
5.87m, then, from 4.36% at 10.09m to 4.40% at 10.87m.
Transmission then remained at approximately 4.40% from
10.87m to 14.35m.

Figure 8.17: CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 6 at 1157
The temperature at station 7 (Figure 8.18) declined from
14.11 oC to 12.81 oC from the
surface to 20.67m. A sharp decrease in temperature was
seen between 3.33m and 5.98m from 14.03 oC to
13.63 oC. Salinity remained steady around
33.75 from the surface to 3.84 m, a rapid increase then
occured to 34.41 at 4.35m. Salinity rose steadily for
the remainder of the water column until a maximum of
35.04 at 20.67m. Transmission remained constant around
4.33% until a depth of 4.6m where it then increased
rapidly to 4.37% at 6.23m. A steady decline in %
transmission with minor fluctuations then occurred until
a minimum value of 4.42% was reached at 20.55m.
Fluorescence declined slightly over the entire water
column from around 0.25V at the surface to 0.16V at
20.67m. As also seen at Station 5, we saw large peaks in
fluorescence at depth. These occurred at 20.55m and
16.99m with voltages of 0.44V and 0.41V respectively,
both of these values were larger than any other in the
entire water column.

Figure 8.18 CTD data
measuring temperature (⁰C), fluorescence (V), salinity
and transmission (%) measured at Station 7 at 1233
CHEMICAL RESULTS OF
ESTUARINE PRACTICAL
At Station 2 of the estuarine
sample set (Figure 9.1), data was recorded from 1m and
3m depths. Between depths of 1 to 3m oxygen saturation
percentages only increased by 1 per cent, from 91% to
92%. Dissolved silicon levels decreased over the depths,
from 28.43µmol/l to 17.36µmol/l. Phosphate levels showed
a relative decrease of 0.149µmol/l, from 0.795µmol/l to
0.647µmol/l over the depth increase.

Figure 9.1: Phospohate
(µmol/L), Silicon (µmol/L) and 02 (% saturation)
profiles at Station 2 at 0945
At Station 3 of the estuarine
sample set (Figure 9.2), data was recorded from 2
different depths, 2m and 9m. Oxygen saturation
percentages decreased over the depth change from 96% to
95% saturated. Dissolved silicon and nitrate levels also
decreased from 21.29µmol/l to 10.08µmol/l and 3.60µmol/l
to 1.61µmol/l consecutively. Only one result for
Phosphate could be recorded due to low levels of sample
solution. The single Phosphate sample showed a value of
0.544µmol/l.

Figure 9.2: Phospohate
(µmol/L), Silicon (µmol/L) and Nitrate (µmol/L) and 02
(% saturation) profiles at Station 3 at 1020
At Station 4 of the estuarine
sample set (Figure 9.3), data was recorded from 2
different depths, 1m and 3m. Oxygen saturation
percentage values did not change between these depths.
Dissolved silicon levels decreased from 13.09µmol/l to
12.60µmol/l with the decrease in depth. Both phosphate
and nitrate levels only have single data points due to
lack of low levels of sample solution and limited
laboratory equipment.

Figure 9.3: Phospohate
(µmol/L), Silicon (µmol/L) and Nitrate (µmol/L) and 02
(% saturation) profiles at Station 4 at 1104
At Station 5 of the estuarine
sample set (Figure 9.4), data was recorded from 5
different depths, 1, 3, 5, 7 and 9m. A slight increase
in oxygen saturation was seen from 1m to 3m from 101% to
104%, and then an intermediate drop was recorded at 5m
to 90% saturated. At 7m another increase in oxygen
saturation was recorded at 101% which decreased to 97%
at 9m. Dissolved silicon levels followed a similar
pattern to oxygen levels, however an increase was
recorded from 11.47µmol/l to 7.89µmol/l at depths of 1m
and 3m. Dissolved silicon levels then increased to
12.60µmol/l at 5m and dropped down to 6.37µmol/l and
5.77µmol/l at 7m and 9m consecutively. Phosphate levels
were recorded for 3, 5, 7 and 9m. Phosphate
concentration increased from 0.191µmol/l to 0.204µmol/l
at depths of 3 and 5m and then decreased to 0.191µmol/l
and 0.166µmol/l depths of 7 and 9m. Only one sample of
nitrate was processed at this station at a depth of 1
metre, the value recorded was 2.20µmol/l.

Figure 9.4: Phospohate
(µmol/L), Silicon (µmol/L) and Nitrate (µmol/L) and 02
(% saturation) profiles at Station 5 at 11.43
At Station 6 of the estuarine
sample set (Figure 9.5), data was recorded from 2
different depths, 2m and 9m. Oxygen saturation
percentage values decreased from 98% saturated to 95%
saturated between the two depths. Dissolved silicon
levels also decreased with increasing depths, from
7.45µmol/l to 3.85µmol/l. Phosphate increased relatively
with an increase in depth, from 0.217µmol/l to
0.332µmol/l.

Figure 9.5: Phospohate
(µmol/L), Silicon (µmol/L) and 02 (% saturation)
profiles at Station 6 at 1157
At Station 7 of the estuarine
sample set (Figure 9.6), data was recorded from 2
different depths, 2m and 9m. Oxygen saturation
percentage values increased from 90% to 98% saturated
between 2 and 9m. Dissolved silicon concentrations
decreased from 4.53µmol/l to 3.13µmol/l with the
increase in water depth. Phosphate levels stayed current
between the two depths at a concentration of
0.114µmol/l.

Figure 9.6: Phospohate
(µmol/L), Silicon (µmol/L) and 02 (% saturation)
profiles at Station 7 at 12.33
THEORETICAL DILUTION LINE
In Figures 9.7-9.9 the theoretical
dilution line (TDL) joins the two end members. The
seawater end member was obtained from the deepest sample
(9.00m) at the last station (7) as this was the highest
salinity sample collected; with a salinity of 34.8 and
silicate concentration of 3.13µmol/l (Figure 9.7)
phosphate concentration of 0.140 µmol/l (Figure 9.8),
and nitrate concentration of 3.31 µmol/l (Figure 9.9) .
The river water end member was calculated by taking an
average of the 3 nutrient concentrations; 87.50, 0.520
and 35.90 µm/l respectively (Figure 1-3) obtained from
the River Allen and Kenwyn, both of which had 0
salinity.

Figure 9.7: Estuarine
mixing diagram showing Silicate concentration against
salinity, the theoretical dilution line joins the 2 end
members

Figure 9.8: Estuarine
mixing diagram showing Phosphate concentration against
salinity, the theoretical dilution line joins the 2 end
members

Figure 9.9: Estuarine
mixing diagram showing Nitrate concentration against
salinity, the theoretical dilution line joins the 2 end
members
The silicate concentrations plotted
on Figure 9.7 generally plot along the theoretical
dilution line (TDL) between the salinity 28.0 and 34.8
towards the mouth of the estuary which is an indication
of conservative behaviour however there are 3 points
which fall either above or below the theoretical
dilution line. A concentration of44.7 µm/l was
calculated at a salinity of 0.3, which fell well below
the TDL suggesting a net removal from the estuary,
similarly to the concentration of 44.60 µm/l recorded at
a salinity of 16.3, however this was only a slight
deviation from the TDL. A silicate concentration of
29.30 µm/l plotted above the TDL suggesting a net gain
of silicate.
In Figure 9.8 a negative
correlation of increased salinity with decreased
phosphate concentration decrease is shown. However, it
appears that phosphate behaved non-conservatively, with
values plotting well above the TDL at intermediate
salinities. For example, at salinity 16.3 the nitrate
concentration is 1.03 µm/l, suggesting that addition of
phosphate is occurring.
The estuarine mixing diagram for
the nitrate shown in Figure 9.9, showed a positive
linear relationship between salinity and nitrate
concentration, as an increase in salinity is reflected
by an increase in nitrate. This relationship and the
fact that the sample points plot along the TDL with
little fluctuation indicates that only nitrate was
behaving conservatively in the estuary, with little
addition or removal occurring.
ESTUARINE RESIDENCE TIME
The residence time of the estuary
was calculated using the equation below, and this was
chosen rather than the tidal prism method because most
of the stations were in the upper Fal estuary where
tidal influences are less dominant.
Tres= ((1- Smean/Ssea)*Vtotal)/R
Where;
Tres= Residence time (s)
Smean= average salinity
Ssea= Salinity of the sea
Vtotal=Total volume of the estuary
(m3)
R=Riverine flux (m3s-1)
The average salinity of 28.84 was
calculated using the underway sample data collected on
3/07/12 (between stations 2 and 5, which were the
transects used to calculate volume) to ensure that the
salinity values used to calculate the average were taken
from the same depth along the estuary and the salinity
of the sea 34.8 was taken at station 7 as this was
approximately the salinity of the sea. The value of the
riverine flux was taken from the Tregony-Fal data
station operated by NERC (www.ceh.ac.uk/datanrfa/data/station.html).
This station covers a catchment area of 87km2 and the
mean flow 0f 2.028 m3s-1 is averaged over the period
1978-2010. The total volume of the estuary was
calculated using both the ADCP data and Google Earth.
The ADCP data was used to work out the cross sectional
area of the horizontal transects (Table 1) however we
only used data from the transects 2,3,4 and 5 as 6 and 7
did not span the full width of the estuary therefore the
data was incomplete. Google Earth was used to plot the
horizontal transects taken at each station (Figure 1)
and the distance between each transect was calculated,
therefore the area could be calculated using the
equation 1/3(a2 + ab + b2)h. The volume calculated of
7005522.83m3 is only a rough approximation as the
calculation assumes that the estuary is straight and the
channel depth increases at a constant rate. Due to the
incomplete ADCP data the full volume of the estuary
could not be calculated and so the residence time
calculated may have been effected.
Tres=((1-28.84/34.8)*7005522.83)/2.028)=591615.6s
= 6.85 days
It should be noted that the
Riverine flux measured at Tregony station may not be a
true representation of the Fal Estuary as it is located
towards the upper Fal River, a great distance from where
the samples were taken and the River Fal is also not the
only freshwater input into the estuary. Secondly the R
value taken was averaged over the period 1978-2010, but
within the month proceeding the sample date there has
been a significant amount of freshwater input from
rainfall therefore due to a combination of factors it is
likely that R is an underestimation and this could have
accounted for the high residence time.
BIOLOGICAL RESULTS OF ESTUARINE PRACTICAL
ZOOPLANKTON
Figure 10.1 indicates that there was a higher abundance
of zooplankton at Trawl C with 248 indivuals per m3 in
total. This is followed by Trawl B at 113 individuals
per m3and then Trawl A with 105 individuals per m3. The
number of species present in trawl B was highest
followed by C then A with 8, 7 and 5 species
respectively. Copepoda Nauplii were most abundant in
all trawls followed by Copepoda spp. Species that were
not present in trawl A that were present in trawls B and
C were Polychaete larvae and Hydromedusae spp.
Cirripedia larvae didn’t appear at all until trawl C.

Figure 10.1:
Zooplankton abundance in cells/m3 from the 3
trawls taken at Stations 2 (trawl A), 5 ( trawl B) and 7
(trawl C)
PHYTOPLANKTON
Phytoplankton abundance was highest at Station 2 (Figure
10.2), not only was there a higher diversity, there was
also a higher number of cells per ml at a total of
39cells. Only R. delicatula, G. flaccida and
R. flaccida were present at both 3m and 1m with
G. flaccida being most abundant over the water
column at 12cells/ml. Thalassiosira spp was most
abundant at 1m at 10cells/ml.

Figure 10.2:
phytoplankton abundance in cells/ml of each recorded
species taken at the sample depths of 3m and 1m
At station 4 (Figure 10.3), no cells were present in our
sample at 3m, however, the number of G. striata
exceeded the number of any cells found at all other
depths and sites with 18cells/ml. Station 7 (Figure
10.4) exhibited the lowest number of cells and the
lowest diversity with only G. flaccida and R.
imbricate at 4 and 3 cells/ml respectively.
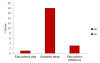
Figure 10.3:
phytoplankton abundance in cells/ml of each recorded
species taken at the sample depths of 3m and 1m
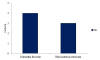
Figure 10.4:
phytoplankton abundance in cells/ml of each recorded
species taken at the sample depth of 9m
CHLOROPHYLL
Figure 10.5 indicated that the highest chlorophyll
concentration recorded was at station 7 at the mouth of
the estuary at 2m depth. The lowest was recorded at
station 6 at 9m depth. At all stations sampled at, with
the exception of station 3, the chlorophyll
concentration recorded decreased with depth. At Station
3, the chlorophyll concentration recorded increased from
4.22 µg/l at 1m depth to 5.22 µg/l at 3m depth. There
appeared to be no clear trend between chlorophyll
concentrations and position along the estuary, more
samples would need to be collected from a range of
positions.

Figure 10.5:
Chlorophyll plotted against depth from station 2 to
station 5
PONTOON REPORT
INTRODUCTION
Date: 05/07/2012
|
station |
Latitude |
longitude |
Time (UTC) |
weather |
|
Pontoon |
50°12.967”N |
005°12.967”W |
08:45-11:15 GMT |
Sunny with periods of rain |
|
|
LW |
HW |
LW |
HW |
|
Time (UTC) |
0031 |
0609 |
1252 |
1826 |
|
Height (m) |
0.3 |
5.0 |
0.3 |
5.3 |
The aim of our research at the pontoon in the Fal
estuary was to collect data for a time series within a
fixed point of the Fal estuary during a tidal cycle. The
equipment we used included a YSI multiprobe and a light
sensor. A YSI multiprobe collects data on the
temperature (°C), salinity, depth (m), acidity (pH),
chlorophyll a (µg/L) and dissolved oxygen (%
saturation). The light sensor detects irradiance levels
in air and water in order to calculate light
attenuation. We were unable to use to current meter due
to technical difficulties.
METHODS
Measurements began at 0845 and were taken every 15
minutes until 1145 to give an idea of changes during the
tidal cycle. Measurements were taken using a YSI
multiprobe and a light sensor. The instruments were
lowered into the water column at 1m intervals, until 4m
depth and then recorded back up the water column as
well. The data below has been plotted in contour plots
to show the parameters in a 2D graphical manner.
YSI
MULTIPROBE
Figure 11.1. Temperature – Between
8:45-11:15 GMT it is clear that the surface waters get
gradually warmer. When we first measured the temperature
at 08:45 the surface waters were 14.6°C. By 11:15°C the
water was 15.2°C. There is also a temperature gradient
between the surface waters and the bottom waters, with
the surface waters being much warmer. The temperature
gradient intensified with time; at 08:45 the surface
waters were 0.2°C warmer than the deeper waters, however
by 11:45 the surface waters were 1.2°C warmer than the
bottom waters.

Figure 11.1:
Temperature (⁰C) plotted with depth showing changes
over time from 08:45-11:15GMT.
Figure 11.2. Salinity – The
salinity at the fixed point in the estuary appeared to
change in layers. At 08:45 the salinity was around 32.5
in the surface layers and was slightly higher in the
bottom waters. By the end of the time series the
salinity had decreased to 30.5 as the time approached
low water (13:35). Throughout the series the deeper
bodies of water remained more saline. This is because
the more saline a body of water is, the greater it’s
density. At the beginning of the time series the
halocline was much more intense with a salinity
difference of around 2.5 units whereas at 11:45 the
difference is around 1.5 units.
 Figure 11.2: Salinity plotted against depth showing
changes over time from 08:45-11:15GMT.
Figure 11.2: Salinity plotted against depth showing
changes over time from 08:45-11:15GMT.
Figure 11.3. – As the tidal cycle
continued it appeared that the water column became more
alkaline. At 08:45 the pH was 8.66 in the surface waters
and by 11:45 it had risen to 8.70. This could also be
because there was a period of rain at 10:15, diluting
the water column and therefore increasing the pH. The pH
was higher and therefore more alkaline in the surface
layers of the water column. However, throughout the
period of time we were sampling the pH only varied by
0.04. There was little difference between the pH of the
surface waters and the bottom waters of the water
column, usually only varying by 0.01pH.

Figure 11.3: Acidity(pH) plotted against depth showing
changes over time from 08:45-11:15 GMT
Figure 11.4 – At the beginning of
the times series the levels of phytoplankton
(chlorophyll a) were greater in the bottom levels of the
water column. However as the day continued and the
temperature and dissolved oxygen levels began to
increase, the levels of chlorophyll a began to rise as
well, especially in the surface layers. In the surface
layers at 08:45 the levels of chlorophyll a were 5.0µg/L
but by 11:15 they had increased to 6.0µg/L. This could
possibly be due to migratory patterns of the
phytoplankton, for example diel migrations.

Figure 11.4:
Chlorophyll a (µg/L) plotted against depth showing
changes over time from 08:45-11:15GMT.
Figure 11.5 – The levels of
dissolved oxygen increased throughout the time series.
At 08:45 the levels of dissolved oxygen were around 95%,
whereas they increased to 97.5% in the surface of the
water by 11:15. At the beginning of the time series the
levels of dissolved oxygen showed little variation
throughout the vertical profile, remaining at 95%
dissolved oxygen. However, by the end of the time series
the levels of dissolved oxygen were greatest in the
surface levels at 97.5% and lowest at the bottom of the
water column, remaining at 95.0% dissolved oxygen. This
is most likely due to changing of the tides aerating the
water column and increased phytoplankton activity (refer
to figure 4), photosynthesising and adding oxygen to the
surrounding water.

Figure 11.5: Dissolved
Oxygen (% saturation) plotted against depth showing
changes over time from 08:45-11:15GMT.
Light Sensor
The contour plot (fig5) for light
intensity shows a high percentage light attenuation at
50-60% in the surface depths above 1m. After 2m depth
the percentage light attenuation decreased rapidly to
less than 10% light attenuation. At 09:45 GMT there was
a heavy rain shower, this is clearly visible at this
time as % light attenuation at the surface is greatly
reduced to less than 20%. This is due to increased
turbidity of the surface water due to rain.
Figure 11.6 shows a decrease in
percentage light attenuation as the ebbing tide
progresses to low tide. At 08:45 the percentage light
attenuation was 62.6% at 0m and at 4m was 4.98%. By
11:15 the percentage light attenuation was 44.84% at 0m
and 4.0% at 4m. When the water level decreased during
ebbing tide the light sensor could only be deployed to
4m because the sea floor was reached. As the ebbing
tides reached low water the velocity of the outgoing
tides increase which may have increased turbidity of the
water column as you move towards low tide. However, as
the current meter was not working on the day this is not
definitive.
 Figure 11.6: A contour plot showing changes in light
attenuation against depth over time from 08:45-11:15GMT
Figure 11.6: A contour plot showing changes in light
attenuation against depth over time from 08:45-11:15GMT
Back to top
GEOPHYSICS
OVERVIEW
The aim for the geophysics investigation was to generate
a benthic habitat survey map with the use of sediment
grabs, an ROV and side scan sonar. However, due to legal
reasons, technical problems with the equipment and the
relatively rough conditions, it was not possible to use
this equipment. Instead we resorted to using a drop
camera and inherited data to produce a habitat map of
the Carrick Roads section of the Fal estuary.
 Geophysics poster
Geophysics poster
Back to top
|