Future extinction for Ehux?
[U Riebesell et al. "Reduced calcification of marine phytoplankton in
response to increased atmospheric CO2", Nature, 407, pg 364-7,
21 September, 2000]
Ehux is a wide-ranging species, found as a member of the phytoplankton
community in almost all oceans (see
biogeography). Given that it blooms in many areas, and many remote
oceans far from land, surely it cannot be seriously affected by human
activities? Well not so, if some recent research is correct at least.
Dissolved Inorganic Carbon System and Calcification
One of the most direct effects humankind is having on Earth's environment is
to pump colossal amounts of carbon dioxide into the atmosphere, which will
severely affect global climate. But it is not the climate effects, but
instead a more direct effect of extra CO2 that may spell trouble for Ehux. A
large part of the extra CO2 added to the atmosphere does not stay there but
instead moves on to cross the sea surface and enter the surface ocean,
raising the level of dissolved carbon there. The extra CO2 has the effect of
acidifying the ocean, lowering the pH. It is this effect which could lead to
problems for Ehux.
The ease with which organisms can form calcium carbonate (CaCO3) structures
such as coccoliths is related to the chemistry of the ocean; more precisely
how readily the dissolved substrates will "jump out of solution" into solid
CaCO3. The substrates for CaCO3 are calcium (Ca) and carbonate (CO3) ions.
If these are present in excess in seawater then that water is said to be
"supersaturated with respect to CaCO3", which means that CaCO3 will
precipitate more or less spontaneously from solution. Or at least it would
do if not for high concentrations of ammonium, phosphate and particularly
magnesium in seawater, which supposedly inhibit CaCO3 formation. Although
the ocean is 5-6 times oversaturated with respect to calcite, spontaneous
inorganic precipitation hardly ever occurs. Extraction of the inhibiting
compounds from the coccolith producing vesicles is thought to be one
mechanism by which coccolithophores manage to precipitate CaCO3.
But nevertheless, if calcium and carbonate concentrations are pushed up high
enough, then eventually the inhibiting effects of other compounds are
overcome and spontaneous inorganic precipitation of CaCO3 takes place. On
the other hand, if concentrations of calcium and/or carbonate were to
decrease, the water would become less super-saturated with respect to CaCO3,
and organisms would have to expend more energy in order to precipitate it.
And below a certain level seawater actually becomes corrosive to CaCO3,
tending to dissolve it once made.
Dissolved inorganic carbon in the ocean exists in three forms: dissolved CO2
gas, bicarbonate (HCO3) and carbonate (CO3) ions. The partitioning between
the three forms is governed by the pH (see
plot). The acidification of the ocean by
anthropogenic CO2 is causing carbonate concentrations everywhere to fall
(decreasing by 30% relative to preindustrial by 2050), as carbon atoms in
carbonate molecules move instead into bicarbonate and/or CO2 molecules, and
is hence making it harder for organisms to synthesize their CaCO3
structures.
Calcifying organisms are in trouble.
Ehux Experiments
Ulf Riebesell and colleagues investigated the importance of this by growing
Ehux and other (non-calcifying) phytoplankton in seawater equilibrated with
high atmospheric CO2 concentrations up to 850 ppmv (pre-industrial = 280,
current = 360 ppmv). While the water they grew their cultures in always
remained supersaturating with respect to CaCO3, it became much less so due
to the high CO2. The results are shown in the images below.
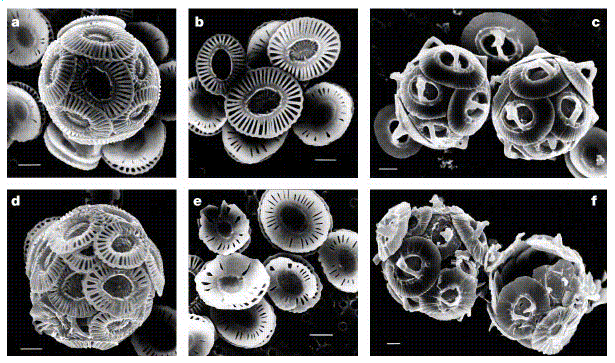
Scanning electron microscopy (SEM) photographs of coccolithophores under
different CO2 concentrations. a, b, d, e:
Emiliania huxleyi; and c, f: Gephyrocapsa oceanica collected
from cultures incubated at CO2 concentration of approx. 12 micro-moles per
litre (a-c) and at CO2 concentration of approx. 30-33 micro-moles per
litre (d-f), corresponding to pCO2 levels of about 300 ppmv and
780-850 ppmv, respectively. Scale bars represent 1 micron. Note the
difference in the coccolith structure (including distinct malformations) and
in the degree of calcification of cells grown at normal and elevated CO2
levels.
Ehux's coccoliths were malformed, presumably because the Ehux cells found it
harder to instigate calcite precipitation within the cell. Since Ehux's
coccoliths presumably serve a useful function (otherwise why produce them),
without them they will probably not be able to compete so well against other
phytoplankton. Any significant penalty could lead to Ehux being replaced by
other phytoplankton in the sea, and hence, eventually, its extinction.
Similar results were obtained for a coral reef in
Biosphere II,
when it too was subjected to high CO2 conditions. The ability to calcify
(form new calcium carbonate) was reduced (Global Biogeochemical
Cycles, 2000, 14, 639-).
So is Ehux destined for the dustbin of history? Will it go extinct in the
future high-CO2 greenhouse? The answer is that this may well happen, but
no-one yet knows for sure.
Past High-CO2 Climates
Despite the general pessimism there is however one ray of hope. If we go far
enough back into the geological past we can eventually find high CO2
climates comparable to the more intense CO2 greenhouse we are presently
creating. We have to go back many millions of years, to the Cretaceous for
instance (~145 to 65 million years ago).
Coccolithophores survived that time (although not Ehux, it hadn't evolved
then), and even fared relatively well in the hot, high-CO2 climate, despite
the possibly low carbonate ion concentrations. We can glean that much at least
from the reasonably abundant coccoliths that fell into in marine sediments
forming at that time. Calcium concentrations were higher during the
Cretaceous and solid calcium carbonate is present in cores from that time,
down to similar depths as to today. This all suggests that the ocean was
probably about equally supersaturated with respect to CaCO3 as it is today,
despite the high CO2.
To sum up, coccolithophores including Ehux may well be in deep trouble in the
decades and centuries ahead, as more and more fossil fuel CO2 diffuses
across the sea-surface and makes the surface ocean more acidic. But let's
hope that future research proves this to be an unfounded worry - it would
indeed be a shame to lose this beautiful natural phenomenon.
Toby Tyrrell, Hanno Kinkel.
In recent years laboratory experiments have been superceded by mesocosm
experiments such as the large multi-institution project described
here.
Ehux
home page
Toby Tyrrell : T.Tyrrell@noc.soton.ac.uk
